Preparation and Properties of Poly(ethylene glycol-co-cyclohexane-1,4-dimethanol terephthalate)/Polyglycolic Acid (PETG/PGA) Blends
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Tensile Testing
2.4. Scanning Electron Microscopy (SEM)
2.5. Differential Scanning Calorimetry (DSC)
2.6. Wide-Angle X-ray Diffraction (WAXD)
2.7. Vicat Softening Temperature (VST)
2.8. Torque
2.9. Melt Mass Flow Rate (MFR)
2.10. Transmission Electron Microscopy (TEM)
3. Results and Discussion
3.1. PETG/PGA Binary Blends
3.1.1. Tensile Performance
3.1.2. Scanning Electron Microscopy (SEM)
3.1.3. DSC and WAXD Measurements
3.1.4. Vicat Softening Temperature (VST)
3.1.5. Melt Mass Flow Rate (MFR)
3.2. PETG/PGA/ADR Ternary Blend
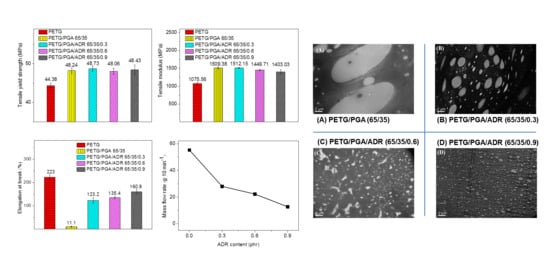
3.2.1. Torque and MFR
3.2.2. Tensile Performance
3.2.3. TEM Observation
3.2.4. SEM Observation
3.2.5. DSC and WAXD Measurements
3.2.6. Vicat Softening Temperature (VST)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tsai, Y.; Fan, C.-H.; Hung, C.-Y.; Tsai, F.-J. Poly(ethylene Terephthalate) Copolymers That Contain 5-Tert-Butylisophthalic Acid and 1-3/1-4-Cyclohexanedimethanol: Synthesis, Characterization, and Properties. J. Appl. Polym. Sci. 2007, 104, 279–285. [Google Scholar] [CrossRef]
- Latko, P.; Dydek, K.; Boczkowska, A. Thermal, Rheological and Mechanical Properties of PETG/RPETG Blends. J. Polym. Environ. 2019, 27, 2600–2606. [Google Scholar] [CrossRef] [Green Version]
- Paszkiewicz, S.; Szymczyk, A.; Irska, I.; Pawlikowska, D.; Piesowicz, E. Synthesis and Characterization of New Reactive Polymer Blends Based on Post-Consumer Glycol-Modified poly(ethylene Terephthalate) Foils and poly(tetramethylene Oxide). Polimery 2018, 63, 45–48. [Google Scholar] [CrossRef]
- Li, B.; Zhang, X.; Zhang, Q.; Chen, F.; Fu, Q. Synergistic Enhancement in Tensile Strength and Ductility of ABS by Using Recycled PETG Plastic. J. Appl. Polym. Sci. 2009, 113, 1207–1215. [Google Scholar] [CrossRef]
- Kováčová, M.; Kozakovičová, J.; Procházka, M.; Janigová, I.; Vysopal, M.; Černičková, I.; Krajčovič, J.; Špitalský, Z. Novel Hybrid PETG Composites for 3D Printing. Appl. Sci. 2020, 10, 3062. [Google Scholar] [CrossRef]
- Bhandari, S.; Lopez-Anido, R.A.; Gardner, D.G. Enhancing the Interlayer Tensile Strength of 3D Printed Short Carbon Fiber Reinforced PETG and PLA Composites via Annealing. Addit. Manuf. 2019, 30, 100922. [Google Scholar] [CrossRef]
- Yu, S.-H.; Lee, J.-h.; Sim, J.-H. Characterization of PETG Thermoplastic Composites Enhanced TiO2, Carbon Black, and POE. Textile Coloration Finish. 2019, 31, 354–362. [Google Scholar] [CrossRef]
- Tsai, Y.; Fan, C.-H.; Hung, C.-Y.; Tsai, F.-J. Transparent copolyester/Organoclay Nanocomposites Prepared by in Situ Intercalation Polymerization: Synthesis, Characterization, and Properties. Polym. Compos. 2010, 32, 89–96. [Google Scholar] [CrossRef]
- Schmidt, C.; Behl, M.; Lendlein, A.; Beuermann, S. Synthesis of High Molecular Weight Polyglycolide in Supercritical Carbon Dioxide. RSC Adv. 2014, 4, 35099–35105. [Google Scholar] [CrossRef] [Green Version]
- Göktürk, E.; Pemba, A.G.; Miller, S.A. Polyglycolic Acid from the Direct Polymerization of Renewable C1 Feedstocks. Polym. Chem. 2015, 6, 3918–3925. [Google Scholar] [CrossRef]
- Samantaray, P.K.; Little, A.; Haddleton, D.M.; McNally, T.; Tan, B.; Sun, Z.; Huang, W.; Ji, Y.; Wan, C. Poly(glycolic Acid) (PGA): A Versatile Building Block Expanding High Performance and Sustainable Bioplastic Applications. Green Chem. 2020, 22, 4055–4081. [Google Scholar] [CrossRef]
- Jem, K.J.; Tan, B. The Development and Challenges of Poly (lactic Acid) and Poly (glycolic Acid). Adv. Ind. Eng. Polym. Res. 2020, 3, 60–70. [Google Scholar] [CrossRef]
- Chang, L.-F.; Zhou, Y.-G.; Ning, Y.; Zou, J. Toughening Effect of Physically Blended Polyethylene Oxide on Polyglycolic Acid. J. Polym. Environ. 2020, 28, 2125–2136. [Google Scholar] [CrossRef]
- Guo, W.; Zhang, Y.; Feng, P.; Gao, C.; Yang, Y.; Yang, W.; Peng, S.; Shuai, C. Montmorillonite With Unique Interlayer Space Imparted Polymer Scaffolds With Sustained Release of Ag+. Ceram. Int. 2019, 45, 11517–11526. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, B.; Xi, Z.; Zhao, L.; Cen, L.; Yang, Y. A Comparable Study of Polyglycolic acid’s Degradation on macrophages’ Activation. Mater. Sci. Eng. C 2020, 109, 110574. [Google Scholar] [CrossRef] [PubMed]
- Lebedev, B.; Yevstropov, A.; Kiparisova, Y.; Belov, V. The Thermodynamics of Glycollide, Polyglycollide and of Polymerization of Glycollide in the Temperature Range of 0–550°K. Polym. Sci. USSR 1978, 20, 32–42. [Google Scholar] [CrossRef]
- Pack, J.W.; Kim, S.H.; Cho, I.-W.; Park, S.Y.; Kim, Y.H. Microstructure Analysis and Thermal Property of Copolymers Made of Glycolide and ƹ-Caprolactone by Stannous Octoate. J. Polym. Sci. Part A Polym. Chem. 2002, 40, 544–554. [Google Scholar] [CrossRef]
- Dutta, A.; Ghosh, A.K. Influence of Thermal and Morphological Characteristics on Mechanical Responses of polypropylene/γ-Irradiated Elastomer Blends. J. Appl. Polym. Sci. 2018, 135, 46597. [Google Scholar] [CrossRef]
- Wang, S.; Pang, S.; Pan, L.; Xu, N.; Li, T. Isothermal Cold Crystallization, Heat Resistance, and Tensile Performance of Polylactide/Thermoplastic Polyester Elastomer (PLA/TPEE) Blends: Effects of Annealing and Reactive Compatibilizer. Polymer 2016, 8, 417. [Google Scholar] [CrossRef] [Green Version]
- Al-Itry, R.; Lamnawar, K.; Maazouz, A. Improvement of Thermal Stability, Rheological and Mechanical Properties of PLA, PBAT and Their Blends by Reactive Extrusion With Functionalized Epoxy. Polym. Degrad. Stab. 2012, 97, 1898–1914. [Google Scholar] [CrossRef]
- Fried, J.R. Polymer Science and Technology; China Edition (original 2nd ed.); PEARSON EDUCATION ASIA LID.: Beijing, China, 2010; pp. 153–205. [Google Scholar]
- Liu, J.; Liu, J. Characterization of Maleic anhydride/Styrene Melt-Grafted Random Copolypropylene and Its Impact on Crystallization and Mechanical Properties of Isotactic Polypropylene. Polym. Bull. 2018, 76, 4369–4387. [Google Scholar] [CrossRef]
- Nagarajan, V.; Mohanty, A.K.; Misra, M. Perspective on Polylactic Acid (PLA) Based Sustainable Materials for Durable Applications: Focus on Toughness and Heat Resistance. ACS Sustain. Chem. Eng. 2016, 4, 2899–2916. [Google Scholar] [CrossRef]
- Song, D.; Wang, K.; Shen, J.; Zhao, L.; Xu, N.; Pang, S.; Pan, L. Effects of Organic Montmorillonite (OMMT) and Pre-Orientation on Property of Poly(l-Lactic Acid) (PLLA)/Ethylene Propylene Diene Monomer (EPDM) Blends. Polymer 2020, 12, 106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zong, X.-H.; Wang, Z.-G.; Hsiao, B.S.; Chu, B.; Zhou, J.J.; Jamiolkowski, D.D.; Muse, E.; Dormier, E. Structure and Morphology Changes in Absorbable Poly(glycolide) and Poly(glycolide-Co-Lactide) During in Vitro Degradation. Macromolecules 1999, 32, 8107–8114. [Google Scholar] [CrossRef]
- Ayyoob, M.; Lee, D.H.; Kim, J.H.; Nam, S.W.; Kim, Y.J. Synthesis of poly(glycolic Acids) via Solution Polycondensation and Investigation of Their Thermal Degradation Behaviors. Fibers Polym. 2017, 18, 407–415. [Google Scholar] [CrossRef]
- Yu, C.; Bao, J.; Xie, Q.; Shan, G.; Pan, P.; Bao, Y. Crystallization Behavior and Crystalline Structural Changes of poly(glycolic Acid) Investigated via Temperature-Variable WAXD and FTIR Analysis. CrystEngComm 2016, 18, 7894–7902. [Google Scholar] [CrossRef]
- Wang, S.; Pang, S.; Xu, N.; Pan, L.; Lin, Q. In Situcompatibilization of polylactide/Thermoplastic Polyester Elastomer Blends Using a Multifunctional Epoxide Compound As a Processing Agent. J. Appl. Polym. Sci. 2016, 133, 43424. [Google Scholar] [CrossRef]
- Härth, M.; Dörnhöfer, A. Film Blowing of Linear and Long-Chain Branched Poly(ethylene Terephthalate). Polymer 2020, 12, 1605. [Google Scholar] [CrossRef]
- Quiles-Carrillo, L.; Montanes, N.; Lagaron, J.M.; Balart, R.; Torres-Giner, S. In Situ Compatibilization of Biopolymer Ternary Blends by Reactive Extrusion With Low-Functionality Epoxy-Based Styrene–Acrylic Oligomer. J. Polym. Environ. 2018, 27, 84–96. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, L.; Han, C.; Yu, Y. Biodegradable Blends of poly(butylene Adipate-Co-Terephthalate) and Stereocomplex Polylactide With Enhanced Rheological, Mechanical Properties and Thermal Resistance. Colloid Polym. Sci. 2020, 298, 1–13. [Google Scholar] [CrossRef]

















| Sample Number | PETG (wt.) | PGA (wt.) | ADR (phr) |
|---|---|---|---|
| 1# | 100 | 0 | 0 |
| 2# | 95 | 5 | 0 |
| 3# | 85 | 15 | 0 |
| 4# | 75 | 25 | 0 |
| 5# | 65 | 35 | 0 |
| 6# | 65 | 35 | 0.3 |
| 7# | 65 | 35 | 0.6 |
| 8# | 65 | 35 | 0.9 |
| Samples | Tensile Yield Strength (MPa) | Tensile Modulus (MPa) | Elongation at Break (%) |
|---|---|---|---|
| Neat PETG | 44.38 ± 0.62 | 1076 ± 35 | 223.0 ± 10.2 |
| PETG/PGA (95/05) | 45.26 ± 0.62 | 1145 ± 17 | 227.2 ± 8.9 |
| PETG/PGA (85/15) | 46.33 ± 0.20 | 1254 ± 07 | 260.4 ± 14.2 |
| PETG/PGA (75/25) | 47.57 ± 0.77 | 1370 ± 50 | 29.0 ± 6.5 |
| PETG/PGA (65/35) | 48.24 ± 0.72 | 1509 ± 31 | 11.1 ± 1.8 |
| Samples | Tg (°C) (PETG) | Tm (°C) (PGA) | ΔHm (J/g) (PGA) | Xc (%) (PGA) |
|---|---|---|---|---|
| Neat PGA | N/A | 224.9 | −74.67 | 37.7 |
| Neat PETG | 74.9 | N/A | N/A | N/A |
| PETG/PGA (95/05) | 74.7 | 221.2 | −2.91 | 29.4 |
| PETG/PGA (85/15) | 74.1 | 221.6 | −9.48 | 31.9 |
| PETG/PGA (75/25) | 72.3 | 221.1 | −17.49 | 35.3 |
| PETG/PGA (65/35) | 73.1 | 221.0 | −24.96 | 36.0 |
| Samples | Tensile Yield Strength (MPa) | Tensile Modulus (MPa) | Elongation at Break (%) |
|---|---|---|---|
| Neat PETG | 44.38 ± 0.62 | 1076 ± 35 | 223.0 ± 10.2 |
| PETG/PGA (65/35) | 48.24 ± 0.72 | 1509 ± 31 | 11.1 ± 1.8 |
| PETG/PGA/ADR (65/35/0.3) | 48.73 ± 0.69 | 1512 ± 15 | 123.2 ± 11.4 |
| PETG/PGA/ADR (65/35/0.6) | 48.06 ± 0.80 | 1450 ± 20 | 135.4 ± 5.1 |
| PETG/PGA/ADR (65/35/0.9) | 48.43 ± 1.28 | 1403 ± 58 | 160.8 ± 9.4 |
| Samples | Tg (°C) (PETG) | Tm (°C) (PGA) | ΔHm (J/g) (PGA) | Xc (%) (PGA) |
|---|---|---|---|---|
| Neat PGA | N/A | 224.9 | −74.67 | 37.7 |
| Neat PETG | 74.9 | N/A | N/A | N/A |
| PETG/PGA (65/35) | 73.1 | 221.0 | −24.96 | 36.0 |
| PETG/PGA/ADR (65/35/0.3) | 73.3 | 220.4 | −22.15 | 32.0 |
| PETG/PGA/ADR (65/35/0.6) | 71.9 | 220.1 | −16.25 | 23.6 |
| PETG/PGA/ADR (65/35/0.9) | 72.6 | 219.9 | −11.28 | 16.4 |
| Samples | VST (°C) |
|---|---|
| Neat PETG | 81.0 |
| PETG/PGA (65/35) | 99.2 |
| PETG/PGA/ADR (65/35/0.3) | 95.0 |
| PETG/PGA/ADR (65/35/0.6) | 94.1 |
| PETG/PGA/ADR (65/35/0.9) | 90.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; Shen, J.; Ma, Z.; Zhang, Y.; Xu, N.; Pang, S. Preparation and Properties of Poly(ethylene glycol-co-cyclohexane-1,4-dimethanol terephthalate)/Polyglycolic Acid (PETG/PGA) Blends. Polymers 2021, 13, 452. https://doi.org/10.3390/polym13030452
Wang K, Shen J, Ma Z, Zhang Y, Xu N, Pang S. Preparation and Properties of Poly(ethylene glycol-co-cyclohexane-1,4-dimethanol terephthalate)/Polyglycolic Acid (PETG/PGA) Blends. Polymers. 2021; 13(3):452. https://doi.org/10.3390/polym13030452
Chicago/Turabian StyleWang, Kai, Jianing Shen, Zhao Ma, Yipeng Zhang, Nai Xu, and Sujuan Pang. 2021. "Preparation and Properties of Poly(ethylene glycol-co-cyclohexane-1,4-dimethanol terephthalate)/Polyglycolic Acid (PETG/PGA) Blends" Polymers 13, no. 3: 452. https://doi.org/10.3390/polym13030452
APA StyleWang, K., Shen, J., Ma, Z., Zhang, Y., Xu, N., & Pang, S. (2021). Preparation and Properties of Poly(ethylene glycol-co-cyclohexane-1,4-dimethanol terephthalate)/Polyglycolic Acid (PETG/PGA) Blends. Polymers, 13(3), 452. https://doi.org/10.3390/polym13030452