1. Introduction
Silver (Ag) is a powerful bactericidal substance that has been used since ancient times. Recent studies have revealed three plausible mechanisms underlying its antimicrobial effects: First, absorption of free Ag ions followed by disruption of ATP production and DNA replication. Second, Ag nanoparticles may release Ag
+, thereby leading to the generation of reactive oxygen species (ROS) that attack microorganisms. Third, Ag nanoparticles directly damage the cell membrane. It is expected that the use of Ag-based antimicrobial materials would help overcome the hurdle of antibiotic resistance encountered with commonly used antibiotics, thereby making Ag the rising star of new antibacterial materials [
1].
Various formulations containing Ag-based compounds have been used in many antimicrobial applications since the nineteenth century. Among them, Ag nanoparticles (AgNPs) have attracted the most attention owing to their unique physical, chemical, and biological properties. AgNP technology has been used in various processes and products [
1]; AgNPs can be used in liquid form, such as gels, suspension coatings, and sprays, added to a solid matrix such as polymeric substrates, or suspended in a solid such as soap. They can also be incorporated into fiber spinning or filtration membranes for water-purification systems [
2]. From the different types of solid matrix material available, electrospun polyacrylonitrile (PAN) has been widely investigated owing to its thermal stability, chemical resistance, and enhanced mechanical properties [
3]. In addition, it has been widely used as a filter material, as well as for nanocomposites and other applications [
4,
5,
6]. PAN is inert, strong, and stable, making it a suitable matrix material for biomaterial production for musculoskeletal systems, such as for promoting bone growth and wound healing [
7].
In the musculoskeletal system, the most frequently noted effects of high-energy trauma (missile, vehicle traffic injury, crush or blast injury, falling from heights) are skin and soft tissue wound defects and even loss of some bony tissue. Considering the limitations of current therapies for bone defect management, bone tissue engineering (BTE) has drawn considerable attention in the past few decades [
8,
9,
10,
11,
12]. Osseous defects caused by segmental bone resection due to tumor, trauma, or infection pose a great challenge for reconstruction and restoration of limb function. BTE focuses on the development of bone regeneration strategies involving loading of osteogenic cells onto various materials. One of the most common approaches is the development of a porous, bone structure–mimicking, three-dimensional (3D) scaffold with appropriate mechanical strength. Various materials such as metals, ceramics, polymers, and carbon-based nanomaterials are used to fabricate BTE scaffolds. Metal scaffolds for BTE applications, which provide superior mechanical properties, have been widely studied in recent years [
13]. However, these scaffolds have several disadvantages, including stress shielding, osteointegration, and toxic metal ion and particle release [
12]. Ceramic-based scaffolds are osteoinductive and biodegradable but are exceedingly brittle and have low mechanical strength [
14]. Polymer-based scaffolds have great potential for use in BTE because of their superior biocompatibility, flexibility, and high biodegradability [
14]. Musculoskeletal injuries often lead to skin and soft tissue wound defects, along with bone fractures. Therefore, while treating bone defects in the musculoskeletal structure, it is important to cover the wound next to the bone defect. With the advances made in biomedical materials, the medical dressings used to cover wounds now differ from traditional dressings such as cotton gauze.
The purpose of this study was to develop a new material with dual modalities for treating musculoskeletal trauma complicated with bone and soft tissue defects with the assistance of nanomaterials. The fine pores and urchin-like Ag–Au bimetallic nanoparticle-decorated block microbial invasion from the outside, and the fibrous PAN nonwoven mat substrate is expected to guide the migration of cell-like osteoblasts to the fracture to heal defects.
2. Materials and Methods
2.1. Materials
PAN (Mw ≈ 150 kDa; 181315-50G; Sigma-Aldrich, St. Louis, MO, USA), Ag nitrate (S8157-10G, >99%, Sigma-Aldrich), l-ascorbic acid (A2174-100G, Sigma-Aldrich), dimethyl sulfoxide (DMSO; ≥99.7%, Sigma Aldrich, St. Louis, MO, USA), Dulbecco’s Modified Eagle Medium (DMEM; 21063-029; 500 mL; Gibco, Grand Island, NY, USA), and thiazol blue tetrazolium bromide (MTT; M2128-1g; 98%; Sigma Aldrich, St. Louis, MO, USA) were used without further purification. N,N-Dimethylformamide (reagent grade; Echo Chemical Co., Ltd., Miaoli, Taiwan) was used as the solvent in the electrospinning process.
2.2. Preparation of Ag-Containing PAN Nonwoven Mat
PAN and AgNO3 were dissolved in DMF and stirred homogenously at room temperature to prepare the stock solution for the subsequent electrospinning process. We used a 1 mL syringe (inner diameter, 4.7 mm) to store the stock solution and attached a 20G needle (inner diameter, 0.6 mm; outer diameter, 0.9 mm; length, 25.4 mm) and then placed this apparatus in the injection pump (New Era Pump Systems, Inc., Syringe Pump NE-300, St. Farmingdale, NY, USA). A 10 × 10 cm2 iron sheet was utilized as an electrically grounded collector. The positive electrode of the high-voltage power supply was clamped on the needle, and the grounded wire was clamped to the iron sheet collector. The parameters were set as follows: voltage, 17 kV; flow rate, 7 μL/min; working distance, 12 cm to maintain a stable corn-jet mode electrospinning. After all the parameters were precisely set and a high-voltage power supply was applied, an electrical field was established between the capillary tube and collection substrate, and the electrospun PAN nanofibers were deposited on the collector. During the electrospinning process, we controlled the humidity of the working environment at approximately 40–50% for the pure PAN nonwoven mat (dehumidifier, Kolin Inc., Taipei, Taiwan) and approximately 20–30% for the AgPAN nonwoven mat by using a dehumidifier.
2.3. Generation of Ag NPs and Their Conversion to Urchin-Like Ag–Au Bimetallic NPs on PAN Nonwoven Mats by Using Galvanic Reaction and Calcined Ag/PAN Nonwoven Mats
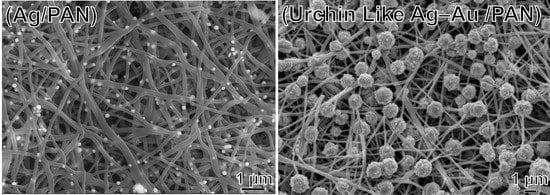
Using ascorbic acid as the reducing agent, the Ag ions trapped in PAN were reduced to Ag NPs. Ascorbic acid (0.32 g) was added to deionized water (25 mL) and then the electrospun AgPAN dressing was soaked in the solution for 2 min to generate army green Ag NPs on an army green PAN nonwoven mat (Ag/PAN).
For the Ag–Au bimetallic NP-decorated PAN nonwoven mat, the abovementioned freshly prepared Ag/PAN was immersed in HAuCl
4 (aq) solution (0.25 g HAuCl
4 dissolved in 25 mL deionized water) for the following galvanic reaction for 1 min:
After the galvanic reaction, the nonwoven mat was placed in an oven (JUN YANG instrument co., LTD., New Taipei city, Taiwan) heated to 37 °C for 1–3 h for production of the urchin-like Ag–Au bimetallic NP-decorated PAN nonwoven mats.
For the production of calcined Ag/PAN nonwoven mats, we placed the AgPAN into the tubular high-temperature furnace (tube Furnace, Strong Youth Co., LTD., Taipei, Taiwan), the atmosphere inside was evacuated to 30 mmHg and filled with nitrogen. We set the heating procedures as follows: heating to 900 °C with rate 5 °C/min and the temperature was held at 900 °C for 1 h. After it was naturally cooled down to room temperature, the sample was taken out. The whole process was carried out at a rate of 50 mL/min under nitrogen gas flow.
2.4. Characterization
The morphological features of the nonwoven mats were observed using a high-resolution field-emission scanning electron microscope (FE-SEM; JSM-6500F; JEOL, Tokyo, Japan). The nanofiber diameters, Ag nanoparticle diameters, and nanofiber porosity were all determined by analyzing the SEM images with the image analysis software ImageJ (NIH, Bethesda, MD, USA). Raman spectra were measured with a commercial Raman microscope (Nanofinder 30, Tokyo Instruments Inc., Tokyo, Japan). The excitation wavelength was 532 nm.
2.5. Measurement of In Vitro Ag-Ion Release
The amount of Ag ions released from the Ag or urchin-like Ag–Au NP-decorated nonwoven mats was determined using inductively coupled plasma-optical emission spectrometry (ICP-OES; iCAP PRO; ThermoFisher Scientific, Waltham, MA, USA). A nonwoven mat piece (25 mg) was placed in a 15 mL vial, and 10 mL distilled water was added to the vial as the elution medium. The vial was shaken at approximately 100 rpm and incubated at 37 °C in a water bath. At the desired time point, all the medium was removed and replaced with 10 mL fresh medium to maintain a constant volume. Subsequently, all of the elution media collected were used for ICP-OES analyses, and the data for the amount of Ag ions released were used to establish a release profile.
2.6. Cytotoxicity of PAN Nonwoven Mats, Ag NPs, and Ag–Au Bimetallic Nanoparticle-Decorated Nonwoven Mats toward 3T3 Cells
To confirm whether Ag or Ag–Au-decorated nonwoven mats were toxic to skin cells, an MTT assay was performed using a mouse fibroblast cell line (NIH-3T3, Bioresource Collection and Research Center, Hsinchu, Taiwan) to test the cytotoxicity of various types of nonwoven mats toward cells in vitro. Basically, an extract solution method was adopted to conduct the MTT assay. Cells (n = 3, 5 × 104/well) were seeded in 24-well plates (Biofil, Guangzhou Jet Bio-Filtration Co., Ltd., Guangzhou, China), and medium was added to obtain a total volume of 1 mL; subsequently, the plates were cultured in an incubator for 24 h (1st well plate). Then, 5 mg of pure PAN, Ag/PAN, and Ag–Au/PAN nonwoven mats was weighed and irradiated with UV light (254 nm) for 30 min sterilization. The same concentration of commercially available nano-Ag suspension kept steady for 24 h was used as the positive control, and medium without soaking of any samples was used as the negative control. The weighed nonwoven mats were immersed in 2 mL serum-free medium in another 24-well plate (2nd well plate) and placed in the incubator (Sanyo Electric Co., Ltd., Gifu, Japan) for 24 h. After 24 h of extraction, the medium in the 1st well plate was removed and the cells were washed twice with 1 mL phosphate-buffered saline (PBS, 10X, UniRegion Bio-tech, Miaoli, Taiwan). Subsequently, 500 μL of the nonwoven mat’s extract solution was taken from the 2nd well plate and added to the cell plate (1st well plate). After the predetermined treatment time, the medium was removed from the plate and MTT solution (thiazol blue tetrazolium bromide (MTT; M2128-1G; 98%; Sigma-Aldrich, 0.5 mg/mL) was added to each well to react with enzyme in the mitochondrial of the cell; the plate was subsequently incubated for 2 h. Then, MTT solution was replaced by DMSO and the plate was incubated for 10 min. Next, absorbance was measured at 570 nm using a UV-Visible spectrophotometer (BMG Labtech, Ortenberg, Germany).
2.7. Evaluation of Antibacterial Effect
The antibacterial effect of various types of nonwoven mats was evaluated using two methods. The first method was the disc diffusion method [
8]. The inhibition zone was analyzed using the qualitative disc diffusion method with 10
9 colony-forming units (CFU) of
Staphylococcus aureus cultured on LB agar plates and placed on 8 mm nonwoven mat samples. The cultured plates were then incubated for 24 h at 37 °C in an incubator, following which the inhibition zone of the microbial colony was observed and measured (OLYMPUS CORPORATION, Tokyo, Japan).
The second method involved quantitative evaluation in a bacterial culture solution test [
9]. The nonwoven mats were cocultured with
S. aureus LB broth suspension. The bacterial solution was sampled from the incubated LB broth at predetermined time points (1, 5, and 24 h) and immediately streaked on nutrient agar (Gibco LB Broth, Liquid, Thermo Fisher Scientific, MA, USA) plates using four-way streak plate inoculation. The plates were then incubated at 37 °C for 16–20 h, and the colonies on the plate were counted for each sample. A plot of colony-forming units (CFU/mL) of
S. aureus versus time was prepared for quantitative analysis of the antibacterial rates for each sample.
2.8. Skin Sensitization Study
TegadermTM film (6 × 7 cm2; 3M Healthcare, Neuss, Germany) was used as the backsheet for immobilizing the nonwoven mat. Pure PAN, Ag/PAN, and Ag–Au/PAN nonwoven mats (size, 1 × 1 cm2) were cut and stuck to the middle of the TegadermTM film. This animal test is consistent with and approved as per the animal use protocol of the Institutional Animal Care and Use Committee (IACUC) of the National Defense Medical Center (NDMC; certificate no. IACUC-17-305). All procedures were in accordance with the ISO 10993-10:2002 tests for irritation and skin sensitization specifications. Our sensitization and irritation tests were thus based on ISO10993-10 guidelines, which suggest suitable animal species and time-period 3 days and 1–3 weeks to see the acute and long-term response of the sensitization and irritation evaluation. To study allergic reactions to the nonwoven mat, guinea pigs with high skin sensitivity were utilized. The dorsal surface of the guinea pigs was shaved before the test, and a 2 × 4 cm2 shaved area was used as the test area for clear observation. In this preclinical animal test, the positive and negative control groups included animals for whom a skin irritant chemical and pure PAN, respectively, had been used. The experimental groups were Ag/PAN and urchin-like Ag–Au/PAN. The test substances for each group were then applied to the test area and covered with 3M dressing (Tegaderm™, St. Paul, MN, USA). We continuously monitored skin sensitivity every day for 7 days long.
2.9. Skin Irritation Study
The skin irritation study was similar to the skin sensitization study, except for the animal species used. A 3M Tegaderm™ film (6 × 7 cm2) was used as a base dressing for adherence to the nonwoven mats. Pure PAN, Ag/PAN, and urchin-like Ag–Au/PAN dressings of appropriate sizes were cut and stuck to the middle of the 3M dressing. The procedures used adhere to the ISO 10993-10:2010 guidelines for irritation and skin sensitization specifications. We applied the test substances directly on the skin surface of the animal for daily observation. In this experiment, New Zealand white rabbits were used as test subjects.
The rabbits were shaved before the test, and a 2 × 4 cm2 area was marked as the targeted test area in the shaved region. The positive and negative control groups included animals for whom a skin irritant chemical and pure PAN, respectively, had been used. The experimental groups were PAN, Ag/PAN, and urchin-like Ag–Au/PAN (n = 3/group). The test dressings of each group were covered in the test area and covered with 3M dressing (Tegaderm™). Skin conditions were observed and recorded after 24, 48, and 72 h.
2.10. In Vivo Evaluation of the Wounded Mouse Model
C57BL/6JNarl mice were used for this analysis due to their thicknesses of full skin are similar to that of human, and their sizes are convenient for animal fixation to conduct full skin removal surgery; the experiment was performed on the dorsal surface of the mice (n = 5/group). Their backs were shaved, and they were anesthetized with ZoletilTM (20–40 mg/kg, Virbac, Carros, France). A 1 × 1 cm2 wound was created on the back of the mice by performing a full skin removal surgery. The previously cultured S. aureus bacterial suspension was diluted to 108 CFU/mL in PBS. Then, 150 μL bacterial suspension was dropped onto the wound by using a micropipette. After 1–3 min, we wrapped the wound with 3M dressings and maintained wound induction in the infected wound model for 1 week. After 7 days of wound infection, the pus on the wound was collected and suspended in 1 mL PBS. The mixture was vibrated for 50 s with an ultrasonic processor and centrifuged at 1000 rpm for 8 min at 4 °C. The supernatant was cultured for 20 h before performing the plate count. After the wound was cleaned and the remaining pus removed, the wound was wrapped with various types of dressings, and the dressing was replaced every 6 days or 2 days for Ag/PAN nonwoven mat and urchin-like Ag–Au/PAN nonwoven mats, respectively, as these two types of dressing show different ability on antibacterial and extrudate absorption ability.
2.11. Pathological Analysis
The healed mice were euthanized, and the regenerated skin area of the dorsal wounds and the liver of the mice were harvested. After the specimens were maintained in formalin for 24 h, they were sent to the Taipei Pathology Center for pathological examination, and Masson, hematoxylin and eosin (H&E) staining was performed. The pathological sections were analyzed using microscopy, and the newborn skin and original skin tissues were compared.
2.12. Culture and Functional Assay of Osteoblasts/Immunofluorescence Staining
The osteoblasts were isolated from 1-day-old newborn rats as previously described [
15]. Briefly, osteoblasts were harvested from calvariae and cultured in α-MEM medium with 10% FBS, 0.05% ascorbic acid (sc-394304, Santa Cruz Biotechnology, Santa Cruz, CA, USA), 2 mM β-glycerophosphate (G9422, Cayman Chemical, Ann Arbor, MI, USA), 10 nM dexamethasone (sc-29059B, Santa Cruz Biotechnology). Medium were changed every 3 days. Osteoblasts of 10
5/cm
2 were seeded into a 6-well plate containing PAN with the same medium for 28 days. Then, osteoblasts were fixed and labeled with primary anti-Core-binding factor alpha1 (Cbfa1, ab23981, Abcam, Cambridge, MA, USA) antibody and FITC-conjugated secondary antibody. Actin was labeled by iFluor™ 546-Phalloidin (22663, AAT Bioquest, Sunnyvale, CA, USA). Cell nuclei were visualized by DAPI staining. Imagines of the fluorescently stain osteoblast cells were taken by using confocal microscope (LSM 880, Zeiss, Oberkochen, Germany).
2.13. Statistical Analyses
Data are expressed as mean ± standard deviation (SD). Statistical analyses were performed using SPSS software (SPSS, Chicago, IL, USA). Specifically, Wilcoxon statistics, one-way analysis of variance, and the Student’s t test were used to assess the differences between the experimental groups. The data were considered significantly different when p < 0.05.