Investigation of the Chemical Structure of Ultra-Thin Polyimide Substrate for the Xenon Flash Lamp Lift-off Technology
Abstract
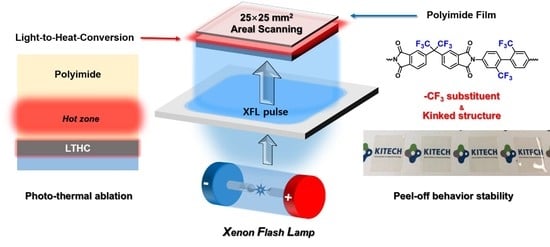
:1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
2.2. Synthesis of Poly(Amic Acid) Solutions
2.2.1. BPI (BPDA-PDA) (BPI-a)
2.2.2. BPI (PMDA-ODA) (BPI-b)
2.2.3. BPI (BPDA-ODA) (BPI-c)
2.2.4. FPI (PMDA-TFDB) (FPI-a)
2.2.5. FPI (PMDA-6FODA) (FPI-b)
2.2.6. FPI (6FDA-TFDB) (FPI-c)
2.3. Fabrication of Polyimide Films
2.4. XF-LO Process Applied to Polyimide Films
3. Results and Discussion
3.1. XF-LO Process Behavior of the Synthesized Polyimide Films
3.2. Investigation of Thermal Stability of Peeled-off Polyimide Films
3.3. Investigation of Optical Properties of Peeled-off Polyimide Films
3.4. Investigation of Film Characteristics According to Thickness
3.5. Investigation of the Surface Characteristics of the Released PI Films
3.6. Investigation of Dielectric Properties of Peeled-off Polyimide Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Mativenga, M.; Geng, D.; Kim, B.; Jang, J. Fully Transparent and Rollable Electronics. ACS Appl. Mater. Interfaces 2015, 7, 1578–1585. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, C.; Yin, Y.; Li, Y.; Xing, Y.; Song, J. Functional Soft Composites As Thermal Protecting Substrates for Wearable Electronics. Adv. Funct. Mater. 2019, 29. [Google Scholar] [CrossRef]
- Kang, Z.; Zhang, Y.; Zhou, M. AgNPs@CNTs/Ag hybrid films on thiolated PET substrate for flexible. Chem. Eng. J. 2019, 368, 223–234. [Google Scholar] [CrossRef]
- Xu, H.; Luo, D.; Li, M.; Xu, M.; Zou, J.; Tao, H.; Lin-Feng, L.; Wang, L.; Peng, J.; Cao, Y. A flexible AMOLED display on the PEN substrate driven by oxide thin-film transistors using anodized aluminium oxide as dielectric. J. Mater. Chem. C 2014, 2, 1255–1259. [Google Scholar] [CrossRef]
- Hanada, T.; Negishi, T.; Shiroishi, I.; Shiro, T. Plastic substrate with gas barrier layer and transparent conductive oxide thin film for flexible displays. Thin Solid Films 2010, 518, 3089–3092. [Google Scholar] [CrossRef]
- Lei, P.-H.; Hsu, C.-M.; Fan, Y.-S. Flexible organic light-emitting diodes on a polyestersulfone (PES) substrate using Al-doped ZnO anode grown by dual-plasma-enhanced metalorganic deposition system. Org. Electron. 2013, 14, 236–249. [Google Scholar] [CrossRef]
- Ghosh, D.S.; Chen, T.L.; Mkhitaryan, V.; Pruneri, V. Ultrathin Transparent Conductive Polyimide Foil Embedding Silver Nanowires. ACS Appl. Mater. Interfaces 2014, 6, 20943–20948. [Google Scholar] [CrossRef]
- Song, C.-H.; Han, C.J.; Ju, B.-K.; Kim, J.-W. Photoenhanced Patterning of Metal Nanowire Networks for Fabrication of Ultraflexible Transparent Devices. ACS Appl. Mater. Interfaces 2016, 8, 480–489. [Google Scholar] [CrossRef]
- Jeong, G.; Koo, D.; Seo, J.; Jung, S.; Choi, Y.; Lee, J.; Park, H. Suppressed Interdiffusion and Degradation in Flexible and Transparent Metal Electrode-Based Perovskite Solar Cells with a Graphene Interlayer. Nano Lett. 2020, 20, 3718–3727. [Google Scholar] [CrossRef]
- Bian, J.; Zhou, L.; Wan, X.; Liu, M.; Zhu, C.; Huang, Y.; Yin, Z. Experimental study of laser lift-off of ultra-thin polyimide film for flexible electronics. Sci. China Technol. Sci. 2019, 62, 233–242. [Google Scholar] [CrossRef]
- Bian, J.; Zhou, L.; Yang, B.; Yin, Z.; Huang, Y. Theoretical and experimental studies of laser lift-off of nonwrinkled ultrathin polyimide film for flexible electronics. Appl. Surf. Sci. 2020, 499, 143910. [Google Scholar] [CrossRef]
- Kim, Y.; Noh, Y.; Park, S.; Kim, B.-K.; Kim, H.J. Ablation of polyimide thin-film on carrier glass using 355 nm and 37 ns laser pulses. Int. J. Heat Mass Transf. 2020, 147, 118896. [Google Scholar] [CrossRef]
- Cozzens, R.F.; Fox, R.B. Infrared laser ablation of polymers. Polym. Eng. Sci. 1978, 18, 900–904. [Google Scholar] [CrossRef]
- Brannon, J.H.; Lankard, J.R.; Baise, A.I.; Burns, F.C.; Kaufman, J.H. Excimer laser etching of polyimide. J. Appl. Phys. 1985, 58, 2036–2043. [Google Scholar] [CrossRef]
- Dyer, P.E.; Jenkins, S.D.; Sidhu, J. Development and origin of conical structures on XeCl laser ablated polyimide. Appl. Phys. Lett. 1986, 49, 453–455. [Google Scholar] [CrossRef]
- Taylor, R.S.; Singleton, D.L.; Paraskevopoulos, G. Effect of optical pulse duration on the XeCl laser ablation of polymers and biological tissue. Appl. Phys. Lett. 1987, 50, 1779–1781. [Google Scholar] [CrossRef]
- Lee, S.I.; Jang, S.H.; Han, Y.J.; Lee, J.Y.; Choi, J.; Cho, K.H. Xenon Flash Lamp Lift-Off Technology without Laser for Flexible Electronics. Micromachines 2020, 11, 953. [Google Scholar] [CrossRef]
- Okada, T.; Ishige, R.; Ando, S. Effects of chain packing and structural isomerism on the anisotropic linear and volumetric thermal expansion behaviors of polyimide films. Polym. 2018, 146, 386–395. [Google Scholar] [CrossRef]
- Li, K.; Liu, Y.; Zheng, H.; Huang, G.; Li, G. Enhanced gas-condensate production by wettability alteration to gas wetness. J. Pet. Sci. Eng. 2011, 78, 505–509. [Google Scholar] [CrossRef]
- Tapaswi, P.K.; Ha, C.-S. Recent Trends on Transparent Colorless Polyimides with Balanced Thermal and Optical Properties: Design and Synthesis. Macromol. Chem. Phys. 2019, 220, 1800313. [Google Scholar] [CrossRef]
- Yi, C.; Li, W.; Shi, S.; He, K.; Ma, P.; Chen, M.; Yang, C. High-temperature-resistant and colorless polyimide: Preparations, properties, and applications. Sol. Energy 2020, 195, 340–354. [Google Scholar] [CrossRef]
- Choi, I.H.; Sohn, B.; Chang, J.-H. Synthesis and characterization of transparent copolyimide films containing CF3 groups: Comparison with copolyimide nanocomposites. Appl. Clay Sci. 2010, 48, 117–126. [Google Scholar] [CrossRef]
- Go, H.; Han, E.-M.; Kang, M.H.; Kim, Y.-H.; Yun, C. The coated porous polyimide layers for optical scattering films. AIMS Mater. Sci. 2017, 5, 1102–1111. [Google Scholar] [CrossRef]
- Yang, Y.; Park, J.H.; Jung, Y.; Lee, S.W.; Kil Park, S.; Kwon, S. Effect of fluorination on haze reduction in transparent polyimide films for flexible substrates. J. Appl. Polym. Sci. 2016, 134, 44375. [Google Scholar] [CrossRef]
- Oliveira, V.M.; Nunes, B.; Vilar, R. Wetting response of KrF laser ablated polyimide surfaces. Nucl. Instrum. Methods Phys. Res. B 2010, 268, 1626–1630. [Google Scholar] [CrossRef]
- Wang, Z.; Zheng, H.; Lim, C.P.; Lam, Y. Polymer hydrophilicity and hydrophobicity induced by femtosecond laser direct irradiation. Appl. Phys. Lett. 2009, 95, 111110. [Google Scholar] [CrossRef]
- Lippert, T. Interaction of Photons with Polymers: From Surface Modification to Ablation. Plasma Process. Polym. 2005, 2, 525–546. [Google Scholar] [CrossRef]
- Brezini, A.; Zekri, N. X-ray photoelectron spectroscopy analysis of polyimide films modified by ultraviolet pulsed laser radiation at 193 nm. J. Appl. Phys. 1994, 75, 2015–2019. [Google Scholar] [CrossRef]
- Lippert, T.; Ortelli, E.; Panitz, J.-C.; Raimondi, F.; Wambach, J.; Wei, J.; Wokaun, A. Imaging-XPS/Raman investigation on the carbonization of polyimide after irradiation at 308 nm. Appl. Phys. A 1999, 69, S651–S654. [Google Scholar] [CrossRef]
- Wolansky, G.; Marmur, A. Apparent contact angles on rough surfaces: The Wenzel equation revisited. Colloids Surf. A Physicochem. Eng. Asp. 1999, 156, 381–388. [Google Scholar] [CrossRef]
- Lv, P.; Dong, Z.; Dai, X.; Wang, H.; Qiu, X. Synthesis and properties of ultralow dielectric porous polyimide films containing adamantane. J. Polym. Sci. A Polym. Chem. 2018, 56, 549–559. [Google Scholar] [CrossRef]
- Tao, L.; Yang, H.; Liu, J.; Fan, L.; Yang, S. Synthesis and characterization of highly optical transparent and low dielectric constant fluorinated polyimides. Polymer 2009, 50, 6009–6018. [Google Scholar] [CrossRef]










| Films | Mean T (%, 500–750 nm) | |||
|---|---|---|---|---|
| XFL Fluence (J/cm2) | ||||
| 0.85 | 2.6 | 4.2 | 5.8 | |
| BPI-a | 90.2 | 85.6 | 81.0 | 71.6 |
| BPI-b | 87.1 | 84.3 | 79.8 | 77.8 |
| FPI-a | 89.5 | 86.6 | 82.1 | 46.2 |
| FPI-b | 88.3 | 88.1 | 86.3 | 85.3 |
| FPI-c | 92.3 | 92.1 | 90.5 | 89.9 |
| Films | Mean T (%, 400–750 nm) | |||
|---|---|---|---|---|
| XFL Fluence (J/cm2) | ||||
| 0.85 | 2.6 | 4.2 | 5.8 | |
| FPI-c-UT (6μm) | 91.7 | 90.7 | 90.0 | 89.2 |
| FPI-c (12μm) | 91.6 | 91.4 | 90.3 | 89.7 |
| FPI-c-TH (30μm) | 92.3 | 92.1 | 91.6 | 90.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, S.H.; Han, Y.J.; Lee, S.Y.; Lee, G.; Jung, J.W.; Cho, K.H.; Choi, J. Investigation of the Chemical Structure of Ultra-Thin Polyimide Substrate for the Xenon Flash Lamp Lift-off Technology. Polymers 2021, 13, 546. https://doi.org/10.3390/polym13040546
Jang SH, Han YJ, Lee SY, Lee G, Jung JW, Cho KH, Choi J. Investigation of the Chemical Structure of Ultra-Thin Polyimide Substrate for the Xenon Flash Lamp Lift-off Technology. Polymers. 2021; 13(4):546. https://doi.org/10.3390/polym13040546
Chicago/Turabian StyleJang, Seong Hyun, Young Joon Han, Sang Yoon Lee, Geonho Lee, Jae Woong Jung, Kwan Hyun Cho, and Jun Choi. 2021. "Investigation of the Chemical Structure of Ultra-Thin Polyimide Substrate for the Xenon Flash Lamp Lift-off Technology" Polymers 13, no. 4: 546. https://doi.org/10.3390/polym13040546
APA StyleJang, S. H., Han, Y. J., Lee, S. Y., Lee, G., Jung, J. W., Cho, K. H., & Choi, J. (2021). Investigation of the Chemical Structure of Ultra-Thin Polyimide Substrate for the Xenon Flash Lamp Lift-off Technology. Polymers, 13(4), 546. https://doi.org/10.3390/polym13040546