Biodegradation of Poly(Lactic Acid) Biocomposites under Controlled Composting Conditions and Freshwater Biotope
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of PLA Films
2.2. Determination of Ultimate Aerobic Biodegradability under Controlled Composting
2.3. Analysis of Biodegradability in Freshwater Biotype
2.3.1. Fourier Transform Infrared Spectroscopy (FT-IR)
2.3.2. Differential Scanning Calorimetry (DSC)
2.3.3. Thermogravimetric Analysis (TGA)
2.3.4. Scanning Electron Microscopy (SEM) Images
3. Results and Discussion
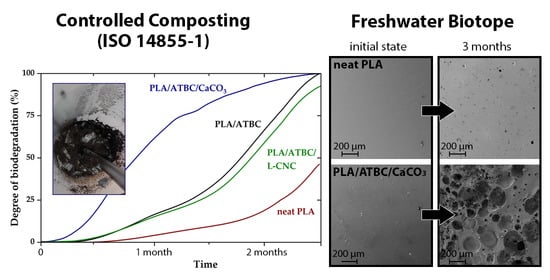
3.1. Determination of Ultimate Aerobic Biodegradability under Controlled Composting
3.2. Analysis of Biodegradability in Freshwater Biotope
3.2.1. Fourier Transform Infrared Spectroscopy (FT-IR)
3.2.2. Differential Scanning Calorimetry (DSC)
3.2.3. Thermogravimetric Analysis (TGA)
3.2.4. Scanning Electron Microscopy (SEM) Images
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thakur, V.K.; Thakur, M.K.; Kessler, M.R. Handbook of Composites from Renewable Materials, Structure and Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2016; Volume 1, ISBN 1-119-22423-3. [Google Scholar]
- Ebnesajjad, S. Handbook of Biopolymers and Biodegradable Plastics: Properties, Processing and Applications; William Andrew: Norwich, NY, USA, 2012; ISBN 1-4557-3003-3. [Google Scholar]
- Maiza, M.; Benaniba, M.T.; Quintard, G.; Massardier-Nageotte, V. Biobased Additive Plasticizing Polylactic Acid (PLA). Polimeros 2015, 25, 581–590. [Google Scholar] [CrossRef]
- Thompson, R.C.; Moore, C.J.; Vom Saal, F.S.; Swan, S.H. Plastics, the Environment and Human Health: Current Consensus and Future Trends. Philos. Trans. Royal Soc. B Biol. Sci. 2009, 364, 2153–2166. [Google Scholar] [CrossRef]
- Kfoury, G.; Raquez, J.-M.; Hassouna, F.; Odent, J.; Toniazzo, V.; Ruch, D.; Dubois, P. Recent Advances in High Performance Poly (Lactide): From “Green” Plasticization to Super-Tough Materials via (Reactive) Compounding. Front. Chem. 2013, 1, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacobsen, S.; Fritz, H.-G. Plasticizing Polylactide—the Effect of Different Plasticizers on the Mechanical Properties. Polym. Eng. Sci. 1999, 39, 1303–1310. [Google Scholar] [CrossRef]
- Rahman, M.; Brazel, C.S. The Plasticizer Market: An Assessment of Traditional Plasticizers and Research Trends to Meet New Challenges. Prog. Polym. Sci. 2004, 29, 1223–1248. [Google Scholar] [CrossRef]
- Vieira, M.G.A.; da Silva, M.A.; dos Santos, L.O.; Beppu, M.M. Natural-Based Plasticizers and Biopolymer Films: A Review. Eur. Polym. J. 2011, 47, 254–263. [Google Scholar] [CrossRef] [Green Version]
- Xiao, H.; Liu, F.; Jiang, T.; Yeh, J.-T. Kinetics and Crystal Structure of Isothermal Crystallization of Poly (Lactic Acid) Plasticized with Triphenyl Phosphate. J. Appl. Polym. Sci. 2010, 117, 2980–2992. [Google Scholar] [CrossRef]
- Burgos, N.; Martino, V.P.; Jiménez, A. Characterization and Ageing Study of Poly (Lactic Acid) Films Plasticized with Oligomeric Lactic Acid. Polym. Degrad. Stab. 2013, 98, 651–658. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, J. Research Progress in Toughening Modification of Poly (Lactic Acid). J. Polym. Sci. Part B Polym. Phys. 2011, 49, 1051–1083. [Google Scholar] [CrossRef]
- Ljungberg, N.; Wesslen, B. Tributyl Citrate Oligomers as Plasticizers for Poly (Lactic Acid): Thermo-Mechanical Film Properties and Aging. Polymer 2003, 44, 7679–7688. [Google Scholar] [CrossRef]
- Tan, B.H.; Muiruri, J.K.; Li, Z.; He, C. Recent Progress in Using Stereocomplexation for Enhancement of Thermal and Mechanical Property of Polylactide. ACS Sustain. Chem. Eng. 2016, 4, 5370–5391. [Google Scholar] [CrossRef]
- Dartora, P.C.; da Rosa Loureiro, M.; de Camargo Forte, M.M. Crystallization Kinetics and Morphology of Poly (Lactic Acid) with Polysaccharide as Nucleating Agent. J. Therm. Anal. Calorim. 2018, 134, 1705–1713. [Google Scholar] [CrossRef]
- Gupta, A.; Simmons, W.; Schueneman, G.T.; Mintz, E.A. Lignin-Coated Cellulose Nanocrystals as Promising Nucleating Agent for Poly (Lactic Acid). J. Therm. Anal. Calorim. 2016, 126, 1243–1251. [Google Scholar] [CrossRef]
- Saeidlou, S.; Huneault, M.A.; Li, H.; Park, C.B. Poly (Lactic Acid) Crystallization. Prog. Polym. Sci. 2012, 37, 1657–1677. [Google Scholar] [CrossRef]
- Samadi, K.; Francisco, M.; Hegde, S.; Diaz, C.A.; Trabold, T.A.; Dell, E.M.; Lewis, C.L. Mechanical, Rheological and Anaerobic Biodegradation Behavior of a Poly (Lactic Acid) Blend Containing a Poly (Lactic Acid)-Co-Poly (Glycolic Acid) Copolymer. Polym. Degrad. Stab. 2019, 170, 109018. [Google Scholar] [CrossRef]
- Aitor, L.; Erlantz, L. A Review on the Thermomechanical Properties and Biodegradation Behaviour of Polyester. Eur. Polym. J. 2019, 121, 1–31. [Google Scholar]
- Siparsky, G.L.; Voorhees, K.J.; Dorgan, J.R.; Schilling, K. Water Transport in Polylactic Acid (PLA), PLA/Polycaprolactone Copolymers, and PLA/Polyethylene Glycol Blends. J. Environ. Polym. Degrad. 1997, 5, 125–136. [Google Scholar]
- Itävaara, M.; Karjomaa, S.; Selin, J.-F. Biodegradation of Polylactide in Aerobic and Anaerobic Thermophilic Conditions. Chemosphere 2002, 46, 879–885. [Google Scholar] [CrossRef]
- Kolstad, J.J.; Vink, E.T.; De Wilde, B.; Debeer, L. Assessment of Anaerobic Degradation of IngeoTM Polylactides under Accelerated Landfill Conditions. Polymer Degrad. Stab. 2012, 97, 1131–1141. [Google Scholar] [CrossRef]
- Karlssion, S.; Ndazi, B.S. Characterization of Hydrolytic Degradation of Polylactic Acid/Rice Hulls Composites in Water at Different Temperatures. Express Polym. Lett. 2011, 5, 119–131. [Google Scholar]
- Kale, G.; Kijchavengkul, T.; Auras, R.; Rubino, M.; Selke, S.E.; Singh, S.P. Compostability of Bioplastic Packaging Materials: An Overview. Macromol. Biosci. 2007, 7, 255–277. [Google Scholar] [CrossRef]
- Cadar, O.; Paul, M.; Roman, C.; Miclean, M.; Majdik, C. Biodegradation Behaviour of Poly (Lactic Acid) and (Lactic Acid-Ethylene Glycol-Malonic or Succinic Acid) Copolymers under Controlled Composting Conditions in a Laboratory Test System. Polym. Degrad. Stab. 2012, 97, 354–357. [Google Scholar] [CrossRef]
- Iovino, R.; Zullo, R.; Rao, M.A.; Cassar, L.; Gianfreda, L. Biodegradation of Poly (Lactic Acid)/Starch/Coir Biocomposites under Controlled Composting Conditions. Polym. Degrad. Stab. 2008, 93, 147–157. [Google Scholar] [CrossRef]
- Soni, R.K.; Soam, S.; Dutt, K. Studies on Biodegradability of Copolymers of Lactic Acid, Terephthalic Acid and Ethylene Glycol. Polym. Degrad. Stab. 2009, 94, 432–437. [Google Scholar] [CrossRef]
- Ren, Y.; Hu, J.; Yang, M.; Weng, Y. Biodegradation Behavior of Poly (Lactic Acid)(PLA), Poly (Butylene Adipate-Co-Terephthalate)(PBAT), and Their Blends Under Digested Sludge Conditions. J. Polym. Environ. 2019, 27, 2784–2792. [Google Scholar] [CrossRef]
- Kallel, T.K.; Houichi, H.; Sayari, A.; Kammoun, M. Assessment of Biodegradation of PLA/PCL and PLA/PEG Biopolymers under Aerobic and Anaerobic Conditions. J. Mater. Environ. Sci. 2015, 6, 2542–2549. [Google Scholar]
- Xu, H.; Yang, Y.; Yu, X. Lightweight Materials from Biopolymers and Biofibers; American Chemical Society: Washington, DC, USA, 2014; ISBN 0-8412-2997-X. [Google Scholar]
- Tuomela, M.; Vikman, M.; Hatakka, A.; Itävaara, M. Biodegradation of Lignin in a Compost Environment: A Review. Bioresour. Technol. 2000, 72, 169–183. [Google Scholar] [CrossRef]
- Micales, J.A.; Skog, K.E. The Decomposition of Forest Products in Landfills. Int. Biodeterior. Biodegrad. 1997, 39, 145–158. [Google Scholar] [CrossRef]
- Hegde, S.; Dell, E.; Lewis, C.; Trabold, T.A.; Diaz, C.A. Anaerobic Biodegradation of Bioplastic Packaging Materials. In Proceedings of the 21st IAPRI World Conference on Packaging, Zhuhai, China, 19–22 June 2018. [Google Scholar]
- Nekhamanurak, Y.B.; Patanathabutr, P.; Hongsriphan, N. Mechanical Properties of Hydrophilicity Modified CaCO3-Poly (Lactic Acid) Nanocomposite. Int. J. Appl. Phys. Math. 2012, 2, 98. [Google Scholar] [CrossRef] [Green Version]
- Nekhamanurak, B.; Patanathabutr, P.; Hongsriphan, N. The Influence of Micro-/Nano-CaCO3 on Thermal Stability and Melt Rheology Behavior of Poly (Lactic Acid). Energy Procedia 2014, 56, 118–128. [Google Scholar] [CrossRef] [Green Version]
- Sessini, V.; Palenzuela, M.; Damián, J.; Mosquera, M.E. Bio-Based Polyether from Limonene Oxide Catalytic ROP as Green Polymeric Plasticizer for PLA. Polymer 2020, 210, 123003. [Google Scholar] [CrossRef]
- Lee, J.C.; Moon, J.H.; Jeong, J.-H.; Kim, M.Y.; Kim, B.M.; Choi, M.-C.; Kim, J.R.; Ha, C.-S. Biodegradability of Poly (Lactic Acid)(PLA)/Lactic Acid (LA) Blends Using Anaerobic Digester Sludge. Macromol. Res. 2016, 24, 741–747. [Google Scholar] [CrossRef]
- Weng, Y.-X.; Wang, L.; Zhang, M.; Wang, X.-L.; Wang, Y.-Z. Biodegradation Behavior of P (3HB, 4HB)/PLA Blends in Real Soil Environments. Polym. Test. 2013, 32, 60–70. [Google Scholar] [CrossRef]
- Amorin, N.S.; Rosa, G.; Alves, J.F.; Gonçalves, S.P.; Franchetti, S.M.; Fechine, G.J. Study of Thermodegradation and Thermostabilization of Poly (Lactide Acid) Using Subsequent Extrusion Cycles. J. Appl. Polym. Sci. 2014, 131, 1–8. [Google Scholar] [CrossRef]
- Zaidi, L.; Kaci, M.; Bruzaud, S.; Bourmaud, A.; Grohens, Y. Effect of Natural Weather on the Structure and Properties of Polylactide/Cloisite 30B Nanocomposites. Polym. Degrad. Stab. 2010, 95, 1751–1758. [Google Scholar] [CrossRef]
- Oliveira, M.; Santos, E.; Araújo, A.; Fechine, G.J.; Machado, A.V.; Botelho, G. The Role of Shear and Stabilizer on PLA Degradation. Polym. Test. 2016, 51, 109–116. [Google Scholar] [CrossRef]
- Wu, H.; Nagarajan, S.; Zhou, L.; Duan, Y.; Zhang, J. Synthesis and Characterization of Cellulose Nanocrystal-Graft-Poly (d-Lactide) and Its Nanocomposite with Poly (l-Lactide). Polymer 2016, 103, 365–375. [Google Scholar] [CrossRef]
- Sasaki, S.; Asakura, T. Helix Distortion and Crystal Structure of the α-Form of Poly (l-Lactide). Macromolecules 2003, 36, 8385–8390. [Google Scholar] [CrossRef]
- Androsch, R.; Schick, C.; Di Lorenzo, M.L. Melting of Conformationally Disordered Crystals (A′-Phase) of Poly (L-lactic Acid). Macromol. Chem. Phys. 2014, 215, 1134–1139. [Google Scholar] [CrossRef]
- Borůvka, M.; Běhálek, L.; Novák, J. Properties and Crystallization of PLLA Biopolymers with Cellulose Nanocrystals and Organic Plasticizer. MM Sci. J. 2020, 2020, 4080–4085. [Google Scholar] [CrossRef]
- Suksut, B.; Deeprasertkul, C. Effect of Nucleating Agents on Physical Properties of Poly (Lactic Acid) and Its Blend with Natural Rubber. J. Polym. Environ. 2011, 19, 288–296. [Google Scholar] [CrossRef]
- Nelson, K.; Retsina, T.; Iakovlev, M.; van Heiningen, A.; Deng, Y.; Shatkin, J.A.; Mulyadi, A. American process: Production of low cost nanocellulose for renewable, advanced materials applications. In Materials Research for Manufacturing; Springer: Berlin/Heidelberg, Germany, 2016; pp. 267–302. [Google Scholar]
- Boruvka, M.; Behalek, L.; Lenfeld, P.; Ngaowthong, C.; Pechociakova, M. Structure-Related Properties of Bionanocomposites Based on Poly (Lactic Acid), Cellulose Nanocrystals and Organic Impact Modifier. Mater. Technol. 2019, 34, 143–156. [Google Scholar] [CrossRef]
- Lizundia, E.; Mateos, P.; Vilas, J.L. Tuneable Hydrolytic Degradation of Poly (l-Lactide) Scaffolds Triggered by ZnO Nanoparticles. Mater. Sci. Eng. C 2017, 75, 714–720. [Google Scholar] [CrossRef] [PubMed]
- Albertsson, A.-C.; Varma, I.K. Aliphatic polyesters: Synthesis, properties and applications. In Degradable Aliphatic Polyesters; Springer: Berlin/Heidelberg, Germany, 2002; pp. 1–40. [Google Scholar]





| Sample Designation | Composition (wt.%) | |||
|---|---|---|---|---|
| PLA | ATBC | L-CNC | CaCO3 | |
| PLA | 100 | - | - | - |
| PLA/ATBC | 90 | 10 | - | - |
| PLA/ATBC/L-CNC | 89 | 10 | 1 | - |
| PLA/ATBC/CaCO3 | 80 | 10 | - | 10 |
| Sample Designation | Proportions (%) |
|---|---|
| PLA | 50.0 |
| ATBC | 59.7 |
| L-CNC | 44.4 |
| CaCO3 | - |
| Sample Designation | pH (-) |
|---|---|
| Blank vessel | 6.5 |
| PLA | 7.0 |
| PLA/ATBC | 7.3 |
| PLA/ATBC/L-CNC | 7.2 |
| PLA/ATBC/CaCO3 | 7.5 |
| Sample Designation | Exposition Time (Months) | 3400 cm−1 | 1747 cm−1 | 1452 cm−1 | 1382 cm−1 | 1359 cm−1 | 1127 cm−1 | 1080 cm−1 |
|---|---|---|---|---|---|---|---|---|
| PLA | Initial state | 0.01 | 0.68 | 0.15 | 0.14 | 0.14 | 0.38 | 0.91 |
| 1 | 0.02 | 0.53 | 0.15 | 0.13 | 0.13 | 0.34 | 0.73 | |
| 2 | 0.02 | 0.38 | 0.11 | 0.10 | 0.10 | 0.27 | 0.56 | |
| 3 | 0.02 | 0.21 | 0.07 | 0.07 | 0.7 | 0.16 | 0.31 | |
| PLA/ATBC | Initial state | 0.01 | 0.68 | 0.15 | 0.14 | 0.14 | 0.39 | 0.89 |
| 1 | 0.02 | 0.57 | 0.15 | 0.13 | 0.13 | 0.36 | 0.77 | |
| 2 | 0.02 | 0.22 | 0.08 | 0.08 | 0.07 | 0.16 | 0.29 | |
| 3 | 0.02 | 0.19 | 0.07 | 0.07 | 0.06 | 0.13 | 0.29 | |
| PLA/ATBC/L-CNC | Initial state | 0.01 | 0.72 | 0.15 | 0.14 | 0.15 | 0.4 | 0.93 |
| 1 | 0.02 | 0.64 | 0.15 | 0.14 | 0.14 | 0.38 | 0.86 | |
| 2 | 0.02 | 0.22 | 0.08 | 0.07 | 0.07 | 0.15 | 0.28 | |
| 3 | 0.03 | 0.14 | 0.07 | 0.08 | 0.06 | 0.12 | 0.22 | |
| PLA/ATBC/CaCO3 | Initial state | 0.01 | 0.54 | 0.24 | 0.15 | 0.14 | 0.35 | 0.73 |
| 1 | 0.02 | 044 | 0.21 | 0.13 | 0.13 | 0.31 | 0.63 | |
| 2 | 0.02 | 0.15 | 0.07 | 0.06 | 0.06 | 0.12 | 0.2 | |
| 3 | 0.02 | 0.08 | 0.05 | 0.04 | 0.04 | 0.07 | 0.12 |
| Sample Designation | Exposition Time (Months) | Tg (°C) | Tcc (°C) | ∆Hcc (J/g) | Tpm (°C) | ∆Hm (J/g) | ∆Hmα’ (J/g) | XC (%) | |
|---|---|---|---|---|---|---|---|---|---|
| PLA | Initial state | 62.0 | 115.7 | 35.62 | 169.2 | 36.3 | - | 0.8 | |
| 1 | 63.1 | 115.2 | 33.98 | 170.6 | 37.7 | - | 4.0 | ||
| 2 | 63.4 | 114.2 | 32.20 | 170.1 | 36.6 | - | - | ||
| 3 | 64.0 | 113.5 | 31.02 | 169.4 | 36.6 | - | - | ||
| PLA/ATBC | Initial state | 46.7 | 104.5 | 22.64 | 143.7 | 152.5 | 26.1 | 16.4 | 4.2 |
| 1 | 37.6 | 87.3 | 20.63 | 139.7 | 151.2 | 25.9 | 9.4 | 6.3 | |
| 2 | 38.8 | 89.3 | 20.94 | 139.1 | 149.7 | 26.4 | 10.0 | - | |
| 3 | 45.1 | 101.5 | 22.78 | 142.8 | 151.8 | 26.0 | 14.6 | - | |
| PLA/ATBC/L-CNC | Initial state | 51.2 | 110.8 | 25.70 | 146.5 | 154.9 | 25.8 | 17.7 | 0.1 |
| 1 | 52.5 | 110.5 | 26.01 | 147.2 | 155.1 | 25.8 | 18.2 | 0.0 | |
| 2 | 52.8 | 110.8 | 26.77 | 147.7 | 155.3 | 26.3 | 18.2 | - | |
| 3 | 51.1 | 109.3 | 26.52 | 146.4 | 154.6 | 25.8 | 16.9 | - | |
| PLA/ATBC/CaCO3 | Initial state | 42.4 | 104.7 | 22.51 | 143.4 | 153.0 | 24.7 | 12.9 | 2.9 |
| 1 | 38.4 | 81.5 | 15.81 | 137.4 | 149.0 | 26.0 | 6.8 | 13.7 | |
| 2 | 37.4 | 78.5 | 15.96 | 140.3 | 150.1 | 22.9 | 7.9 | - | |
| 3 | 46.7 | 93.8 | 19.15 | 141.2 | 151.4 | 22.4 | 9.1 | - | |
| Sample Designation | Exposition Time (Months) | |||||||
|---|---|---|---|---|---|---|---|---|
| Initial State | 1 | 2 | 3 | |||||
| T5 (%) | T50 (%) | T5 (%) | T50 (%) | T5 (%) | T50 (%) | T5 (%) | T50 (%) | |
| PLA | 329.2 | 361.9 | 326.8 | 360.5 | 326.7 | 360.0 | 319.1 | 353.9 |
| PLA/ATBC | 294.3 | 364.3 | 282.2 | 359.9 | 274.5 | 357.8 | 250.9 | 356.8 |
| PLA/ATBC/L-CNC | 312.9 | 364.9 | 312.4 | 361.2 | 309.7 | 359.1 | 308.1 | 355.6 |
| PLA/ATBC/CaCO3 | 267.8 | 320.7 | 247.1 | 299.9 | 233.8 | 299.5 | 233.5 | 299.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brdlík, P.; Borůvka, M.; Běhálek, L.; Lenfeld, P. Biodegradation of Poly(Lactic Acid) Biocomposites under Controlled Composting Conditions and Freshwater Biotope. Polymers 2021, 13, 594. https://doi.org/10.3390/polym13040594
Brdlík P, Borůvka M, Běhálek L, Lenfeld P. Biodegradation of Poly(Lactic Acid) Biocomposites under Controlled Composting Conditions and Freshwater Biotope. Polymers. 2021; 13(4):594. https://doi.org/10.3390/polym13040594
Chicago/Turabian StyleBrdlík, Pavel, Martin Borůvka, Luboš Běhálek, and Petr Lenfeld. 2021. "Biodegradation of Poly(Lactic Acid) Biocomposites under Controlled Composting Conditions and Freshwater Biotope" Polymers 13, no. 4: 594. https://doi.org/10.3390/polym13040594
APA StyleBrdlík, P., Borůvka, M., Běhálek, L., & Lenfeld, P. (2021). Biodegradation of Poly(Lactic Acid) Biocomposites under Controlled Composting Conditions and Freshwater Biotope. Polymers, 13(4), 594. https://doi.org/10.3390/polym13040594