Injectable Hydrogels: From Laboratory to Industrialization
Abstract
:Objectives and List of Content
1. Introduction
2. Injectable Hydrogels: Properties and Synthesis Techniques
3. Characterization Techniques
3.1. Physicochemical Characterization
3.1.1. Gelation Time
3.1.2. Rheology
- i.
- Determine the viscosity of hydrogels as a function of shear rate by a flux shear rate sweep. In this case, a shear thinning behavior of the hydrogels must be observed to confirm the injectability of the hydrogels, unlike non-crosslinked HA solutions that show a Newtonian behavior.
- ii.
- Calculate viscosity values as a function of time at constant shear rate (e.g., 1 s−1) by a flux time sweep. In this case, viscosity values must maintain almost constant over the time without fluctuations to accept the measure.
- iii.
- Before G′ and G″ modulus determination, an oscillatory strain sweep test must be performed in order to know the linear viscoelastic region (LVR) in which concrete strain must be selected and fixed for the subsequent oscillatory frequency sweeps.
- iv.
- Finally, after the previous assessment and the appropriate selection fixing a certain strain (e.g., 1%), elastic (G′) and viscous (G″) modulus can be measured correctly by oscillatory frequency sweeps.
3.1.3. Syringeability and Injectability Evaluation
3.1.4. Spectroscopy and Spectrometry Techniques
3.1.5. Swelling Ability
3.1.6. Stability and Degradation
3.1.7. Other Physicochemical Characterization Techniques
3.2. Structural/Morphological Characterization
3.3. Thermal and Mechanical Characterization
3.4. Biological Characterization
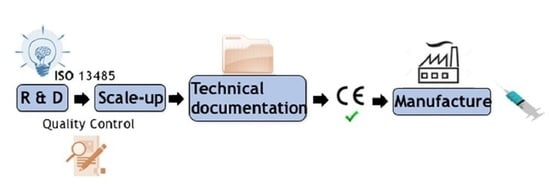
4. Process from the Hydrogel Obtention in the Lab to Its Industrial Production
4.1. Scale-Up Key Technical Parameters: Design and Development
4.1.1. Rheological Parameters
4.1.2. Process Parameters
Dispersing Machinery
Time
Temperature
Purification of the Hydrogel
Filling of Syringes with Hydrogels
4.2. In-House Example: Fabrication of an Injectable Hydrogel for Dermal Filling Applications
4.2.1. Lab-Scale Fabrication
4.2.2. Development Batches
4.2.3. Pilot Batches
4.3. Regulatory Aspects
- Determination of the regulation that applies, considering that hydrogels are active implantable medical devices.
- Classify the medical device: Class I, IIa, IIb, or III. Hydrogels are Class III.
- Implementation of the Quality Management System.
- Elaboration of a technical file with all the available information, including physical, chemical, and technical characteristics and properties, and clinical data about the hydrogel in order to prove its compliance with the regulation.
- Audit from a Notified Body of the QMS and technical file of the hydrogel. The clinical evaluation reports and the post-marketing surveillance activities must be also performed.
- Obtaining the CE marking certificate (valid for three years) for the hydrogel and an ISO 13485 certificate (valid for one year) for the manufacturer facilities. In some countries, a manufacture license is also required.
- Elaboration of a declaration of conformity declaring the compliance of the hydrogel to the corresponding regulation, where the CE marking certificate can be now attached.
- Registration of hydrogels in those member states where their national regulation requests it. The process will be repeated once the CE mark/ISO 13485 certificate loses its validity.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Caló, E.; Khutoryanskiy, V.V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef] [Green Version]
- Qiu, H.; Guo, H.; Li, D.; Hou, Y.; Kuang, T.; Ding, J. Intravesical Hydrogels as Drug Reservoirs. Trends Biotechnol. 2020, 38, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yu, J.; Ren, K.; Zuo, J.; Ding, J.; Chen, X. Thermosensitive Hydrogels as Scaffolds for Cartilage Tissue Engineering. Biomacromolecules 2019, 20, 1478–1492. [Google Scholar] [CrossRef] [PubMed]
- Maiz-Fernández, S.; Pérez-álvarez, L.; Ruiz-Rubio, L.; Vilas-Vilela, J.L.; Lanceros-Mendez, S. Polysaccharide-based in situ self-healing hydrogels for tissue engineering applications. Polymers (Basel) 2020, 12, 2261. [Google Scholar] [CrossRef]
- Balakrishnan, B.; Banerjee, R. Biopolymer-Based Hydrogels for Cartilage Tissue Engineering. Chem. Rev. 2011, 111, 4453–4474. [Google Scholar] [CrossRef] [PubMed]
- Lin-Gibson, S.; Bencherif, S.; Cooper, J.A.; Wetzel, S.J.; Antonucci, J.M.; Vogel, B.M.; Horkay, F.; Washburn, N.R. Synthesis and characterization of PEG dimethacrylates and their hydrogels. Biomacromolecules 2004, 5, 1280–1287. [Google Scholar] [CrossRef] [PubMed]
- Vasi, A.M.; Popa, M.I.; Butnaru, M.; Dodi, G.; Verestiuc, L. Chemical functionalization of hyaluronic acid for drug delivery applications. Mat. Sci. Eng. 2014, 38, 177–185. [Google Scholar] [CrossRef]
- Rice, K.G. The Chemistry, Biology, and Medical Applications of Hyaluronan and Its Derivatives; Laurent, T.C., Ed.; Portland Press: London, UK, 1998. [Google Scholar]
- Piantanida, E.; Alonci, G.; Bertucci, A.; De Cola, L. Design of Nanocomposite Injectable Hydrogels for Minimally Invasive Surgery. Acc. Chem. Res. 2019, 52, 2101–2112. [Google Scholar] [CrossRef]
- Salzlechner, C.; Haghighi, T.; Huebscher, I.; Walther, A.R.; Schell, S.; Gardner, A.; Undt, G.; da Silva, R.M.P.; Dreiss, C.A.; Fan, K.; et al. Adhesive Hydrogels for Maxillofacial Tissue Regeneration Using Minimally Invasive Procedures. Adv. Healthc. Mater. 2020, 9. [Google Scholar] [CrossRef]
- Hyun, H.; Park, M.H.; Lim, W.; Kim, S.Y.; Jo, D.; Jung, J.S.; Jo, G.; Um, S.; Lee, D.W.; Yang, D.H. Injectable visible light-cured glycol chitosan hydrogels with controlled release of anticancer drugs for local cancer therapy in vivo: A feasible study. Artif. Cells Nanomed. Biotechnol. 2018, 46, 874–882. [Google Scholar] [CrossRef] [Green Version]
- 13. Pertici, V.; Pin-Barre, C.; Rivera, C.; Pellegrino, C.; Laurin, J.; Gigmes, D.; Trimaille, T. Degradable and Injectable Hydrogel for Drug Delivery in Soft Tissues. Biomacromolecules 2019, 20, 149–163. [Google Scholar] [CrossRef]
- Ren, B.; Chen, X.; Du, S.; Ma, Y.; Chen, H.; Yuan, G.; Li, J.; Xiong, D.; Tan, H.; Ling, Z.; et al. Injectable polysaccharide hydrogel embedded with hydroxyapatite and calcium carbonate for drug delivery and bone tissue engineering. Inter. J. Bio. Macrom. 2018, 118, 1257–1266. [Google Scholar] [CrossRef]
- Choi, W.I.; Hwang, Y.; Sahu, A.; Min, K.; Sung, D.; Tae, G.; Chang, J.H. An injectable and physical levan-based hydrogel as a dermal filler for soft tissue augmentation. Biomater. Sci. 2018, 6, 2627–2638. [Google Scholar] [CrossRef]
- Collins, M.N.; Birkinshaw, C. Hyaluronic acid based scaffolds for tissue engineering—A review. Carbohydr. Polym. 2013, 92, 1262–1279. [Google Scholar] [CrossRef] [PubMed]
- Deyhle, H.; Schulz, G.; Bert, M. Encyclopedia of Nanotechnology—Injectable Hydrogels; Springer Netherlands: Dordrecht, The Netherlands, 2012; ISBN 9789048197514. [Google Scholar]
- Uman, S.; Dhand, A.; Burdick, J.A. Recent advances in shear-thinning and self-healing hydrogels for biomedical applications. J. Appl. Polym. Sci. 2020, 137, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Mellati, A.; Akhtari, J. Injectable Hydrogels: A Review of Injectability Mechanisms and Biomedical Applications. Res. Mol. Med. 2019, 6, 1–19. [Google Scholar] [CrossRef]
- Loebel, C.; Rodell, C.B.; Chen, M.H.; Burdick, J.A. Shear-thinning and self-healing hydrogels as injectable therapeutics and for 3D-printing. Nat. Protoc. 2017, 12, 1521–1541. [Google Scholar] [CrossRef]
- Le, T.M.D.; Jung, B.K.; Li, Y.; Duong, H.T.T.; Nguyen, T.L.; Hong, J.W.; Yun, C.O.; Lee, D.S. Physically crosslinked injectable hydrogels for long-term delivery of oncolytic adenoviruses for cancer treatment. Biomater. Sci. 2019, 7, 4195–4207. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H. Injectable hydrogels delivering therapeutic agents for disease treatment and tissue engineering. Biomater. Res. 2018, 22, 1–14. [Google Scholar] [CrossRef] [Green Version]
- La Gatta, A.; Schiraldi, C.; Papa, A.; De Rosa, M. Comparative analysis of commercial dermal fillers based on crosslinked hyaluronan: Physical characterization and in vitro enzymatic degradation. Polym. Degrad. Stab. 2011, 96, 630–636. [Google Scholar] [CrossRef]
- Buck, D.W.; Alam, M.; Kim, J.Y.S. Injectable fillers for facial rejuvenation: A review. J. Plast. Reconstr. Aesthetic Surg. 2009, 62, 11–18. [Google Scholar] [CrossRef]
- Kim, Z.H.; Lee, Y.; Kim, S.M.; Kim, H.; Yun, C.K.; Choi, Y.S. A composite dermal filler comprising cross-linked hyaluronic acid and human collagen for tissue reconstructions. J. Microbiol. Biotechnol. 2014, 25, 399–406. [Google Scholar] [CrossRef] [Green Version]
- Moeinzadeh, S.; Jabbari, E. Gelation characteristics, physico-mechanical properties and degradation kinetics of micellar hydrogels. Eur. Polym. J. 2015, 72, 566–576. [Google Scholar] [CrossRef] [Green Version]
- Chung, Y.M.; Simmons, K.L.; Gutowska, A.; Jeong, B. Sol-Gel transition temperature of PLGA-g-PEG aqueous solutions. Biomacromolecules 2002, 3, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Maiz-Fernández, S.; Guaresti, O.; Pérez-Álvarez, L.; Ruiz-Rubio, L.; Gabilondo, N.; Vilas-Vilela, J.L.; Lanceros-Mendez, S. β-Glycerol phosphate/genipin chitosan hydrogels: A comparative study of their properties and diclofenac delivery. Carbohydr. Polym. 2020, 248, 116811. [Google Scholar] [CrossRef]
- Ketabat, F.; Karkhaneh, A.; Mehdinavaz Aghdam, R.; Hossein Ahmadi Tafti, S. Injectable conductive collagen/alginate/polypyrrole hydrogels as a biocompatible system for biomedical applications. J. Biomater. Sci. Polym. Ed. 2017, 28, 794–805. [Google Scholar] [CrossRef] [PubMed]
- Dodero, A.; Williams, R.; Gagliardi, S.; Vicini, S.; Alloisio, M.; Castellano, M. A micro-rheological and rheological study of biopolymers solutions: Hyaluronic acid. Carbohydr. Polym. 2019, 203, 349–355. [Google Scholar] [CrossRef]
- Gloria, A.; Borzacchiello, A.; Causa, F.; Ambrosio, L. Rheological characterization of hyaluronic acid derivatives as injectable materials toward nucleus pulposus regeneration. J. Biomater. Appl. 2012, 26, 745–759. [Google Scholar] [CrossRef] [PubMed]
- Stocks, D.; Sundaram, H.; Michaels, J.; Durrani, M.J.; Wortzman, M.S.; Nelson, D.B. Rheological Evaluation of the Physical Properties. J. Drugs Dermatology 2011, 10, 974–980. [Google Scholar]
- Vanderhooft, J.L.; Alcoutlabi, M.; Magda, J.J.; Prestwich, G.D. Rheological properties of cross-linked hyaluronan-gelatin hydrogels for tissue engineering. Macromol. Biosci. 2009, 9, 20–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertasa, M.; Dodero, A.; Alloisio, M.; Vicini, S.; Riedo, C.; Sansonetti, A.; Scalarone, D.; Castellano, M. Agar gel strength: A correlation study between chemical composition and rheological properties. Eur. Polym. J. 2020, 123, 109442. [Google Scholar] [CrossRef]
- Zuidema, J.M.; Rivet, C.J.; Gilbert, R.J.; Morrison, F.A. A protocol for rheological characterization of hydrogels for tissue engineering strategies. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 1063–1073. [Google Scholar] [CrossRef] [PubMed]
- Ornell, K.J.; Lozada, D.; Phan, N.V.; Coburn, J.M. Controlling methacryloyl substitution of chondroitin sulfate: Injectable hydrogels with tunable long-term drug release profiles. J. Mater. Chem. B 2019, 7, 2151–2161. [Google Scholar] [CrossRef]
- Chiesa, E.; Genta, I.; Dorati, R.; Modena, T.; Conti, B. Poly (gamma-glutamic acid) based thermosetting hydrogels for injection: Rheology and functional parameters evaluation. React. Funct. Polym. 2019, 140, 93–102. [Google Scholar] [CrossRef]
- Moreira, C.D.F.; Carvalho, S.M.; Sousa, R.G.; Mansur, H.S.; Pereira, M.M. Nanostructured chitosan/gelatin/bioactive glass in situ forming hydrogel composites as a potential injectable matrix for bone tissue engineering. Mater. Chem. Phys. 2018, 218, 304–316. [Google Scholar] [CrossRef]
- Maiz-Fernández, S.; Pérez-álvarez, L.; Ruiz-Rubio, L.; González, R.P.; Sáez-Martínez, V.; Pérez, J.R.; Vilas-Vilela, J.L. Synthesis and characterization of covalently crosslinked pH-responsive hyaluronic acid nanogels: Effect of synthesis parameters. Polymers (Basel) 2019, 11, 742. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.; Wang, X.; Li, Y.; Lei, M.; Du, Y.; Kennedy, J.F.; Knill, C.J. Production and characterisation of novel injectable chitosan/ methylcellulose/salt blend hydrogels with potential application as tissue engineering scaffolds. Carbohydr. Polym. 2010, 82, 833–841. [Google Scholar] [CrossRef]
- Ahmad, U.; Sohail, M.; Ahmad, M.; Minhas, M.U.; Khan, S.; Hussain, Z.; Kousar, M.; Mohsin, S.; Abbasi, M.; Shah, S.A.; et al. Chitosan based thermosensitive injectable hydrogels for controlled delivery of loxoprofen: Development, characterization and in-vivo evaluation. Int. J. Biol. Macromol. 2019, 129, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Jin, X.; Li, H.; Zhang, R.R.; Wu, C.W. Injectable and body temperature sensitive hydrogels based on chitosan and hyaluronic acid for pH sensitive drug release. Carbohydr. Polym. 2018, 186, 82–90. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Zhang, D.; Ma, S.; Zhang, J.; Gao, F.; Guan, F.; Yao, M. Dual-enzymatically crosslinked and injectable hyaluronic acid hydrogels for potential application in tissue engineering. RSC Adv. 2020, 10, 2870–2876. [Google Scholar] [CrossRef] [Green Version]
- Rohani Rad, E.; Vahabi, H.; Formela, K.; Saeb, M.R.; Thomas, S. Injectable poloxamer/graphene oxide hydrogels with well-controlled mechanical and rheological properties. Polym. Adv. Technol. 2019, 30, 2250–2260. [Google Scholar] [CrossRef]
- Wende, F.J.; Gohil, S.; Nord, L.I.; Helander, A.; Sandström, C. 1D NMR methods for determination of degree of cross-linking and BDDE substitution positions in HA hydrogels. Carbohydr. Polym. 2017, 157, 1525–1530. [Google Scholar] [CrossRef] [Green Version]
- Pawar, G.M.; Koenigs, M.; Fahimi, Z.; Cox, M.; Voets, I.K.; Wyss, H.M.; Sijbesma, R.P. Injectable hydrogels from segmented PEG-bisurea copolymers. Biomacromolecules 2012, 13, 3966–3976. [Google Scholar] [CrossRef]
- Peers, S.; Montembault, A.; Ladavière, C. Chitosan hydrogels for sustained drug delivery. J. Control. Release 2020, 326, 150–163. [Google Scholar] [CrossRef]
- Sun, Y.; Nan, D.; Jin, H.; Qu, X. Recent advances of injectable hydrogels for drug delivery and tissue engineering applications. Polym. Test. 2020, 81, 106283. [Google Scholar] [CrossRef]
- Holback, H.; Yeo, Y.; Park, K. Hydrogel Swelling Behavior and its Biomedical Applications; Woodhead Publishing Limited: Sawston-Cambridge, UK, 2011. [Google Scholar]
- Kamath, K.R.; Park, K. Biodegradable hydrogels in drug delivery. Adv. Drug Deliv. Rev. 1993, 11, 59–84. [Google Scholar] [CrossRef]
- Li, L.; Wang, N.; Jin, X.; Deng, R.; Nie, S.; Sun, L.; Wu, Q.; Wei, Y.; Gong, C. Biodegradable and injectable in situ cross-linking chitosan-hyaluronic acid based hydrogels for postoperative adhesion prevention. Biomaterials 2014, 35, 3903–3917. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, L.N.D.M.; Franz-Montan, M.; Breitkreitz, M.C.; da Silva, G.H.R.; de Castro, S.R.; Guilherme, V.A.; de Araújo, D.R.; de Paula, E. Nanohybrid hydrogels designed for transbuccal anesthesia. Int. J. Nanomed. 2018, 13, 6453–6463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kureha, T.; Hayashi, K.; Ohira, M.; Li, X.; Shibayama, M. Dynamic Fluctuations of Thermoresponsive Poly(oligo-ethylene glycol methyl ether methacrylate)-Based Hydrogels Investigated by Dynamic Light Scattering. Macromolecules 2018, 51, 8932–8939. [Google Scholar] [CrossRef]
- Sapsford, K.E.; Tyner, K.M.; Dair, B.J.; Deschamps, J.R.; Medintz, I.L. Analyzing nanomaterial bioconjugates: A review of current and emerging purification and characterization techniques. Anal. Chem. 2011, 83, 4453–4488. [Google Scholar] [CrossRef]
- Mingyu, G.; Ming, J.; Pispas, S.; Wei, Y.; Chixing, Z. Supramolecular hydrogels made of End-Functionalized Low-Molecular-Weight PEG and α-Cyclodextrin and their hybridization with SiO2 nanoparticles through Host-Guest interaction. Macromolecules 2008, 41, 9744–9749. [Google Scholar] [CrossRef]
- Li, Q.; Yang, D.; Ma, G.; Xu, Q.; Chen, X.; Lu, F.; Nie, J. Synthesis and characterization of chitosan-based hydrogels. Int. J. Biol. Macromol. 2009, 44, 121–127. [Google Scholar] [CrossRef]
- Mohamed, N.A.; Fahmy, M.M. Synthesis and antimicrobial activity of some novel cross-linked chitosan hydrogels. Int. J. Mol. Sci. 2012, 13, 11194–11209. [Google Scholar] [CrossRef] [PubMed]
- Eid, M. Gamma Radiation Synthesis and Characterization of Starch Based Polyelectrolyte Hydrogels Loaded Silver Nanoparticles. J. Inorg. Organomet. Polym. Mater. 2011, 21, 297–305. [Google Scholar] [CrossRef]
- Waters, D.J.; Engberg, K.; Parke-Houben, R.; Hartmann, L.; Ta, C.N.; Toney, M.F.; Frank, C.W. Morphology of photopolymerized end-linked poly(ethylene glycol) hydrogels by small-angle X-ray scattering. Macromolecules 2010, 43, 6861–6870. [Google Scholar] [CrossRef] [Green Version]
- Bozoğlan, B.K.; Duman, O.; Tunç, S. Preparation and characterization of thermosensitive chitosan/carboxymethylcellulose/scleroglucan nanocomposite hydrogels. Int. J. Biol. Macromol. 2020, 162, 781–797. [Google Scholar] [CrossRef]
- Singh, R.; Hawkins, W. Sutures, ligatures and knots. Surgery (Oxford) 2017, 35, 185–189. [Google Scholar] [CrossRef]
- de Jonge, N.; Houben, L.; Dunin-Borkowski, R.E.; Ross, F.M. Resolution and aberration correction in liquid cell transmission electron microscopy. Nat. Rev. Mater. 2019, 4, 61–78. [Google Scholar] [CrossRef]
- Kaberova, Z.; Karpushkin, E.; Nevoralová, M.; Vetrík, M.; Šlouf, M.; Dušková-Smrcková, M. Microscopic structure of swollen hydrogels by scanning electron and light microscopies: Artifacts and reality. Polymers (Basel) 2020, 12, 578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aston, R.; Sewell, K.; Klein, T.; Lawrie, G.; Grøndahl, L. Evaluation of the impact of freezing preparation techniques on the characterisation of alginate hydrogels by cryo-SEM. Eur. Polym. J. 2016, 82, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Belmar, L.; Toledo, L.; Sánchez, S.A.; Urbano, B.F. Fluorescent nanotubes in PHEMA hydrogels: Visualizing aggregation and distribution by confocal fluorescence microscopy. Mater. Today Commun. 2018, 16, 285–292. [Google Scholar] [CrossRef]
- Stach, S.; Ţălu, Ş.; Trabattoni, S.; Tavazzi, S.; Głuchaczka, A.; Siek, P.; Zając, J.; Giovanzana, S. Morphological Properties of Siloxane-Hydrogel Contact Lens Surfaces. Curr. Eye Res. 2017, 42, 498–505. [Google Scholar] [CrossRef]
- Chang, C.; Duan, B.; Cai, J.; Zhang, L. Superabsorbent hydrogels based on cellulose for smart swelling and controllable delivery. Eur. Polym. J. 2010, 46, 92–100. [Google Scholar] [CrossRef]
- Barroso, N.; Guaresti, O.; Pérez-Álvarez, L.; Ruiz-Rubio, L.; Gabilondo, N.; Vilas-Vilela, J.L. Self-healable hyaluronic acid/chitosan polyelectrolyte complex hydrogels and multilayers. Eur. Polym. J. 2019, 120, 109268. [Google Scholar] [CrossRef]
- Shiblee, M.N.I.; Ahmed, K.; Khosla, A.; Kawakami, M.; Furukawa, H. 3D printing of shape memory hydrogels with tunable mechanical properties. Soft Matter 2018, 14, 7809–7817. [Google Scholar] [CrossRef] [PubMed]
- Al-Sabah, A.; Burnell, S.E.A.; Simoes, I.N.; Jessop, Z.; Badiei, N.; Blain, E.; Whitaker, I.S. Structural and mechanical characterization of crosslinked and sterilised nanocellulose-based hydrogels for cartilage tissue engineering. Carbohydr. Polym. 2019, 212, 242–251. [Google Scholar] [CrossRef]
- Thambi, T.; Phan, V.H.G.; Lee, D.S. Stimuli-Sensitive Injectable Hydrogels Based on Polysaccharides and Their Biomedical Applications. Macromol. Rapid Commun. 2016, 37, 1881–1896. [Google Scholar] [CrossRef]
- Cascone, S.; Lamberti, G. Hydrogel-based commercial products for biomedical applications: A review. Int. J. Pharm. 2020, 573, 118803. [Google Scholar] [CrossRef]
- Almeida, L.D.F.; Babo, P.S.; Silva, C.R.; Rodrigues, M.T.; Hebling, J.; Reis, R.L.; Gomes, M.E. Hyaluronic acid hydrogels incorporating platelet lysate enhance human pulp cell proliferation and differentiation. J. Mater. Sci. Mater. Med. 2018, 29. [Google Scholar] [CrossRef] [Green Version]
- Catanzano, O.; D’Esposito, V.; Acierno, S.; Ambrosio, M.R.; De Caro, C.; Avagliano, C.; Russo, P.; Russo, R.; Miro, A.; Ungaro, F.; et al. Alginate-hyaluronan composite hydrogels accelerate wound healing process. Carbohydr. Polym. 2015, 131, 407–414. [Google Scholar] [CrossRef]
- Ninan, N.; Forget, A.; Shastri, V.P.; Voelcker, N.H.; Blencowe, A. Antibacterial and Anti-Inflammatory pH-Responsive Tannic Acid-Carboxylated Agarose Composite Hydrogels for Wound Healing. ACS Appl. Mater. Interfaces 2016, 8, 28511–28521. [Google Scholar] [CrossRef]
- Reeve, L.; Baldrick, P. Biocompatibility assessments for medical devices–evolving regulatory considerations. Expert Rev. Med. Devices 2017, 14, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.X.; Amidon, G.; Khan, M.A.; Hoag, S.W.; Polli, J.; Raju, G.K.; Woodcock, J. Understanding pharmaceutical quality by design. AAPS J. 2014, 16, 771–783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dacoba, T.G.; Ruiz-Gatón, L.; Benito, A.; Klein, M.; Dupin, D.; Luo, M.; Menta, M.; Teijeiro-Osorio, D.; Loinaz, I.; Alonso, M.J.; et al. Technological challenges in the preclinical development of an HIV nanovaccine candidate. Drug Deliv. Transl. Res. 2020, 10, 621–634. [Google Scholar] [CrossRef]
- Lu, H.D.; Charati, M.B.; Kim, I.L.; Burdick, J.A. Injectable shear-thinning hydrogels engineered with a self-assembling Dock-and-Lock mechanism. Biomaterials 2012, 33, 2145–2153. [Google Scholar] [CrossRef] [PubMed]
- Abdelgawad, K.; Elkatatny, S.; Moussa, T.; Mahmoud, M.; Patil, S. Real-time determination of rheological properties of spud drilling fluids using a hybrid artificial intelligence technique. J. Energy Resour. Technol. Trans. ASME 2019, 141. [Google Scholar] [CrossRef]
- Townsend, J.M.; Beck, E.C.; Gehrke, S.H.; Berkland, C.J.; Detamore, M.S. Flow behavior prior to crosslinking: The need for precursor rheology for placement of hydrogels in medical applications and for 3D bioprinting. Prog. Polym. Sci. 2019, 91, 126–140. [Google Scholar] [CrossRef] [PubMed]
- Catoira, M.C.; González-Payo, J.; Fusaro, L.; Ramella, M.; Boccafoschi, F. Natural hydrogels R&D process: Technical and regulatory aspects for industrial implementation. J. Mater. Sci. Mater. Med. 2020, 31. [Google Scholar] [CrossRef]
- Le Cardinal, G.; Germain, E.; Gelus, M.; Guillon, B. 63 The design of stirred batch polymerisation reactor. Chem. Eng. Sci. 1980, 35. [Google Scholar] [CrossRef]
- Scale-Up in Chemical Engineering, 2nd ed.; Zlokamik, M. (Ed.) WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2006; ISBN 3527314210. [Google Scholar]
- Chien, D.C.H.; Penlidis, A. On-Line Sensors for Polymerization Reactors. J. Macromol. Sci., Part C Polymer Rev. 1990, 30, 1–42. [Google Scholar] [CrossRef]








| Parameters | Formula | Definition and Characteristics |
|---|---|---|
| Viscosity (η) | Strain rate η = Stress | It is the flow characteristic of a gel and it is used to define gel thickness. |
| Elastic viscosity (η′) | η′ is proportional to G″ | |
| Viscous viscosity (η”) | η″ is proportional to G′ | |
| Complex viscosity (η*) | It is the viscosity calculated from frequency sweep. | |
| Elastic modulus (G′) | It characterized the stored energy in a viscoelastic material. Higher G′ values correlates with a firmer gel. | |
| Viscous modulus (G″) | It measures the resistance to dynamic forces. Lower G″ values are less ticker gels and require less force to extrude through a needle. | |
| Complex modulus (G*) | It characterized the overall ability to resist de formation. Injectable gels possess G* equal to G′ | |
| Loss factor (tan δ) | Loss factor measures the relative proportions of elastic to viscous modulus. Hydrogels with low loss factor (close to 0) are predominantly elastic. |
| Difficulties in Scaling up | Suggested Solutions |
|---|---|
| Rheological parameters | Rheological fluid behavior must be normalized to foresee the flow behaviors by comparison with the common standardization function (master curve) and to predict non-Newtonian fluid parameters based on Newtonian models |
| Mixing/dispersing of the hydrogel precursor | Adjust the propeller/dispersor configuration |
| Process time | Perform dimensional analysis based on Reynolds and Archimedes numbers |
| Process temperature | Sensors and software to control temperature depending chemical processes |
| Purification of the hydrogel | Comercial solution for scaling up purification steps |
| Filling of syringes with the hydrogel | Check the rheological properties of the pre-sterilized hydrogel in order to select the filling machinery |
| ISO Standard | Characterization Test |
|---|---|
| ISO 10993-3:2018 | Biological evaluation of medical devices. Part 3: Genotoxicity and carcinogenicity |
| ISO 10993-5:2018 | Biological evaluation of medical devices. Part 5: Cytotoxicity |
| ISO 10993-6:2017 | Biological evaluation of medical devices. Part 6: Tests for local effects after implantation |
| ISO 10993-10:2018 | Biological evaluation of medical devices. Part 10: Tests for irritation and skin sensitization |
| ISO 10993-11:2018 | Biological evaluation of medical devices. Part 11: Tests for systemic toxicity |
| UNE-EN ISO 11607-1:2017 | Packaging for terminally sterilized medical devices. Part 1: Requirements for materials, sterile barrier systems, and packaging systems |
| UNE-EN ISO 11607-2:2017 | Sterilization of medical devices—Microbiological methods. Part 2: Tests of sterility performed in the definition, validation, and maintenance of a sterilization process |
| UNE-EN ISO 11737-1:2018 | Sterilization of health care products—Microbiological methods. Part 1: Determination of a population of microorganisms on product |
| UNE-EN ISO 11737-2:2010 | Sterilization of medical devices—Microbiological methods. Part 2: Tests of sterility performed in the definition, validation, and maintenance of a sterilization process |
| UNE-EN ISO 13485:2018 | Medical devices. Quality management systems. Requirements for regulatory purposes |
| UNE-EN ISO 14630:2013 | Non-active surgical implants. General requirements |
| UNE EN ISO 14644-1: 2016 | Cleanrooms and associated controlled environments. Part 1: Classification of air cleanliness by particle concentration |
| UNE-EN ISO 14644-4:2001 | Cleanrooms and associated controlled environments. Part 4: Design, construction, and start-up |
| ISO 14971:2019 | Medical devices. Application of risk management to medical devices. |
| UNE-EN ISO 15223-1:2017 | Medical devices. Symbols to be used with medical device labels, labelling and information to be supplied. Part 1: General requirements |
| UNE-EN ISO 17665-1:2007 | Sterilization of health care products—Moist heat. Part 1: Requirements for the development, validation, and routine control of a sterilization process for medical devices |
| UNE-EN 62366-1:2015 | Medical devices. Part 1: Application of usability engineering to medical devices |
| UNE-ISO 2859-1:2012 | Sampling procedures for inspection by attributes. Part 1: Sampling schemes indexed by acceptance quality limit (AQL) for lot-by-lot inspection |
| GMP. Annex 1. | Manufacture of Sterile Medicinal Products |
| ISO 16061:2015* *when needles are included | Instrumentation for use in association with non-active surgical implants. General requirements |
| UNE-ISO 2859-1:2012 | Sampling procedures for inspection by attributes. Part 1: Sampling schemes indexed by acceptance quality limit (AQL) for lot-by-lot inspection |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alonso, J.M.; Andrade del Olmo, J.; Perez Gonzalez, R.; Saez-Martinez, V. Injectable Hydrogels: From Laboratory to Industrialization. Polymers 2021, 13, 650. https://doi.org/10.3390/polym13040650
Alonso JM, Andrade del Olmo J, Perez Gonzalez R, Saez-Martinez V. Injectable Hydrogels: From Laboratory to Industrialization. Polymers. 2021; 13(4):650. https://doi.org/10.3390/polym13040650
Chicago/Turabian StyleAlonso, Jose Maria, Jon Andrade del Olmo, Raul Perez Gonzalez, and Virginia Saez-Martinez. 2021. "Injectable Hydrogels: From Laboratory to Industrialization" Polymers 13, no. 4: 650. https://doi.org/10.3390/polym13040650
APA StyleAlonso, J. M., Andrade del Olmo, J., Perez Gonzalez, R., & Saez-Martinez, V. (2021). Injectable Hydrogels: From Laboratory to Industrialization. Polymers, 13(4), 650. https://doi.org/10.3390/polym13040650