Fabricating Antibacterial and Antioxidant Electrospun Hydrophilic Polyacrylonitrile Nanofibers Loaded with AgNPs by Lignin-Induced In-Situ Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Fabrication of PAN Nanofibers via Electrospinning
2.2.2. Synthesis of AgNPs on the Surface of PAN Nanofibers
2.3. Characterizations
2.3.1. Morphological Analysis
2.3.2. Physicochemical Analysis
2.3.3. WAXRD Analysis
2.3.4. Elemental Analysis
2.3.5. Surface Wetting Properties
2.3.6. Swelling Ratio and Yield Measurements of PAN/AgNPs
- WS = Weight of swollen sample, g.
- Wd = Dry weight of the sample after swelling and drying, g.
- W = Dry weight of the sample before swelling, g.
2.3.7. Thermal Analysis
2.3.8. Antibacterial Evaluation of PAN/AgNPs
2.3.9. Ag Contents and Ag Release Kinetics
2.3.10. Antioxidant Activity
2.3.11. Statistical Analysis
3. Results and Discussion
3.1. Morphology of Nanofibers
3.2. FT-IR Spectral Analysis
3.3. WAXRD Analysis
3.4. XPS Analysis
3.5. Surface Wetting Properties
3.6. Swelling Behavior and Yield (%)
3.7. Thermogravimetric Analysis
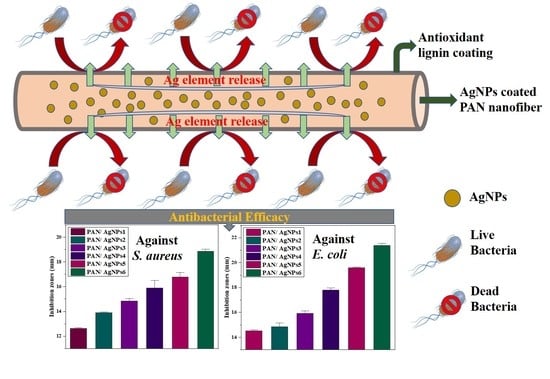
3.8. Antibacterial Evaluation of PAN/AgNPs
3.9. Ag Content and Ag Release Profile
3.10. Antioxidant Activity Analysis
3.11. Practical Applications and Future Research Perspective
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Greiner, A.; Wendorff, J.H. Electrospinning: A fascinating method for the preparation of ultrathin fibers. Angew. Chemie Int. Ed. 2007, 46, 5670–5703. [Google Scholar] [CrossRef]
- Li, D.; Xia, Y. Electrospinning of nanofibers: Reinventing the wheel? Adv. Mater. 2004, 16, 1151–1170. [Google Scholar] [CrossRef]
- Gibson, P.; Lee, C. Application of nanofiber technology to nonwoven thermal insulation. J. Eng. Fibers Fabr. 2007, 2, 155892500700200204. [Google Scholar] [CrossRef] [Green Version]
- Rujitanaroj, P.; Pimpha, N.; Supaphol, P. Wound-dressing materials with antibacterial activity from electrospun gelatin fiber mats containing silver nanoparticles. Polymer (Guildf.) 2008, 49, 4723–4732. [Google Scholar] [CrossRef]
- Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.H.; Park, S.J.; Lee, H.J.; Kim, S.H.; Park, Y.K.; Park, Y.H.; Hwang, C.Y.; et al. Antimicrobial effects of silver nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2007. [Google Scholar] [CrossRef] [PubMed]
- Phan, D.N.; Dorjjugder, N.; Khan, M.Q.; Saito, Y.; Taguchi, G.; Lee, H.; Mukai, Y.; Kim, I.S. Synthesis and attachment of silver and copper nanoparticles on cellulose nanofibers and comparative antibacterial study. Cellulose 2019, 26, 6629–6640. [Google Scholar] [CrossRef]
- Liang, D.; Hsiao, B.S.; Chu, B. Functional electrospun nanofibrous scaffolds for biomedical applications. Adv. Drug Deliv. Rev. 2007, 59, 1392–1412. [Google Scholar] [CrossRef] [Green Version]
- Jin, L.; Zheng, Y.; Liu, Z.; Li, J.; Zhai, H.; Chen, Z.; Li, Y. Design of an Ultrasensitive Flexible Bend Sensor Using a Silver-Doped Oriented Poly (vinylidene fluoride) Nanofiber Web for Respiratory Monitoring. ACS Appl. Mater. Interfaces 2020, 12, 1359–1367. [Google Scholar] [CrossRef]
- Lin, Q.; Li, Y.; Yang, M. Polyaniline nanofiber humidity sensor prepared by electrospinning. Sens. Actuators B Chem. 2012, 161, 967–972. [Google Scholar] [CrossRef]
- Barhate, R.S.; Ramakrishna, S. Nanofibrous filtering media: Filtration problems and solutions from tiny materials. J. Memb. Sci. 2007, 296, 1–8. [Google Scholar] [CrossRef]
- Lee, S.; Obendorf, S.K. Developing protective textile materials as barriers to liquid penetration using melt-electrospinning. J. Appl. Polym. Sci. 2006, 102, 3430–3437. [Google Scholar] [CrossRef]
- Phan, D.N.; Dorjjugder, N.; Saito, Y.; Taguchi, G.; Lee, H.; Lee, J.S.; Kim, I.S. The mechanistic actions of different silver species at the surfaces of polyacrylonitrile nanofibers regarding antibacterial activities. Mater. Today Commun. 2019, 21, 100622. [Google Scholar] [CrossRef]
- Xu, X.; Yang, Q.; Wang, Y.; Yu, H.; Chen, X.; Jing, X. Biodegradable electrospun poly(l-lactide) fibers containing antibacterial silver nanoparticles. Eur. Polym. J. 2006, 42, 2081–2087. [Google Scholar] [CrossRef]
- Saratale, R.G.; Karuppusamy, I.; Saratale, G.D.; Pugazhendhi, A.; Kumar, G.; Park, Y.; Ghodake, G.S.; Bharagava, R.N.; Banu, J.R.; Shin, H.S. A comprehensive review on green nanomaterials using biological systems: Recent perception and their future applications. Colloids Surf. B Biointerfaces 2018, 170, 20–35. [Google Scholar] [CrossRef] [PubMed]
- Oloffs, A.; Grosse-Siestrup, C.; Bisson, S.; Rinck, M.; Rudolph, R.; Gross, U. Biocompatibility of silver-coated polyurethane catheters and silvercoated Dacron® material. Biomaterials 1994, 15, 753–758. [Google Scholar] [CrossRef]
- Rai, M.; Yadav, A.; Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef]
- Saratale, R.G.; Saratale, G.D.; Shin, H.S.; Jacob, J.M.; Pugazhendhi, A.; Bhaisare, M.; Kumar, G. New insights on the green synthesis of metallic nanoparticles using plant and waste biomaterials: Current knowledge, their agricultural and environmental applications. Environ. Sci. Pollut. Res. 2018, 25, 10164–10183. [Google Scholar] [CrossRef] [PubMed]
- Kharaghani, D.; Kee Jo, Y.; Khan, M.Q.; Jeong, Y.; Cha, H.J.; Kim, I.S. Electrospun antibacterial polyacrylonitrile nanofiber membranes functionalized with silver nanoparticles by a facile wetting method. Eur. Polym. J. 2018, 108, 69–75. [Google Scholar] [CrossRef]
- Castellano, J.J.; Shafii, S.M.; Ko, F.; Donate, G.; Wright, T.E.; Mannari, R.J.; Payne, W.G.; Smith, D.J.; Robson, M.C. Comparative evaluation of silver-containing antimicrobial dressings and drugs. Int. Wound J. 2007, 4. [Google Scholar] [CrossRef]
- Yang, Z.; Peng, H.; Wang, W.; Liu, T. Crystallization behavior of poly(ε-caprolactone)/layered double hydroxide nanocomposites. J. Appl. Polym. Sci. 2010, 116, 2658–2667. [Google Scholar] [CrossRef]
- Yeo, S.Y.; Lee, H.J.; Jeong, S.H. Preparation of nanocomposite fibers for permanent antibacterial effect. J. Mater. Sci. 2003, 38, 2143–2147. [Google Scholar] [CrossRef]
- Khan, M.Q.; Kharaghani, D.; Nishat, N.; Shahzad, A.; Hussain, T.; Kim, K.O.; Kim, I.S. The fabrications and characterizations of antibacterial PVA/Cu nanofibers composite membranes by synthesis of Cu nanoparticles from solution reduction, nanofibers reduction and immersion methods. Mater. Res. Express 2019, 6. [Google Scholar] [CrossRef]
- Kharaghani, D.; Lee, H.; Ishikawa, T.; Nagaishi, T.; Kim, S.H.; Kim, I.S. Comparison of fabrication methods for the effective loading of Ag onto PVA nanofibers. Text. Res. J. 2019, 89, 625–634. [Google Scholar] [CrossRef]
- De Santa Maria, L.C.; Santos, A.L.C.; Oliveira, P.C.; Barud, H.S.; Messaddeq, Y.; Ribeiro, S.J.L. Synthesis and characterization of silver nanoparticles impregnated into bacterial cellulose. Mater. Lett. 2009, 63, 797–799. [Google Scholar] [CrossRef]
- Luong, N.D.; Lee, Y.; Nam, J. Do Highly-loaded silver nanoparticles in ultrafine cellulose acetate nanofibrillar aerogel. Eur. Polym. J. 2008, 44, 3116–3121. [Google Scholar] [CrossRef]
- Dong, X.; Ji, X.; Wu, H.; Zhao, L.; Li, J.; Yang, W. Shape control of silver nanoparticles by stepwise citrate reduction. J. Phys. Chem. C 2009, 113, 6573–6576. [Google Scholar] [CrossRef]
- Kumar, A.; Chhatra, R.K.; Pandey, P.S. Synthesis of click bile acid polymers and their application in stabilization of silver nanoparticles showing iodide sensing property. Org. Lett. 2010, 12, 24–27. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Saifullah; Ahmad, M.; Swami, B.L.; Ikram, S. Green synthesis of silver nanoparticles using Azadirachta indica aqueous leaf extract. J. Radiat. Res. Appl. Sci. 2016, 9, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Bar, H.; Bhui, D.K.; Sahoo, G.P.; Sarkar, P.; Pyne, S.; Misra, A. Green synthesis of silver nanoparticles using seed extract of Jatropha curcas. Colloids Surf. A Physicochem. Eng. Asp. 2009, 348, 212–216. [Google Scholar] [CrossRef]
- Hemmati, S.; Rashtiani, A.; Zangeneh, M.M.; Mohammadi, P.; Zangeneh, A.; Veisi, H. Green synthesis and characterization of silver nanoparticles using Fritillaria flower extract and their antibacterial activity against some human pathogens. Polyhedron 2019, 158, 8–14. [Google Scholar] [CrossRef]
- Behravan, M.; Hossein Panahi, A.; Naghizadeh, A.; Ziaee, M.; Mahdavi, R.; Mirzapour, A. Facile green synthesis of silver nanoparticles using Berberis vulgaris leaf and root aqueous extract and its antibacterial activity. Int. J. Biol. Macromol. 2019, 124, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Philip, D.; Unni, C.; Aromal, S.A.; Vidhu, V.K. Murraya Koenigii leaf-assisted rapid green synthesis of silver and gold nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 78, 899–904. [Google Scholar] [CrossRef] [PubMed]
- Bar, H.; Bhui, D.K.; Sahoo, G.P.; Sarkar, P.; De, S.P.; Misra, A. Green synthesis of silver nanoparticles using latex of Jatropha curcas. Colloids Surf. A Physicochem. Eng. Asp. 2009, 339, 134–139. [Google Scholar] [CrossRef]
- Lima, M.J.A.; Reis, B.F. Photogeneration of silver nanoparticles induced by UV radiation and their use as a sensor for the determination of chloride in fuel ethanol using a flow-batch system. Talanta 2019, 201, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, N.; Jing, M.; Zhang, Y.; Wang, W.; Liu, L.; Xu, Z.; Liu, L.; Li, F.; Wu, N. Monodispersed and spherical silver nanoparticles/graphene nanocomposites from gamma-ray assisted in-situ synthesis for nitrite electrochemical sensing. Electrochim. Acta 2019, 295, 434–443. [Google Scholar] [CrossRef]
- Supriya; Kaspate, R.; Pal, C.K.; Sengupta, S.; Basu, J.K. Microwave-mediated synthesis of silver nanoparticles on various metal-alginate composites: Evaluation of catalytic activity and thermal stability of the composites in solvent-free acylation reaction of amine and alcohols. SN Appl. Sci. 2020, 2, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Menazea, A.A.; Abdelghany, A.M.; Osman, W.H.; Hakeem, N.A.; El-Kader, F.H.A. Precipitation of silver nanoparticles in silicate glasses via Nd:YAG nanosecond laser and its characterization. J. Non. Cryst. Solids 2019, 513, 49–54. [Google Scholar] [CrossRef]
- Ma, C.; Li, Z.; Li, J.; Fan, Q.; Wu, L.; Shi, J.; Song, Y. Lignin-based hierarchical porous carbon nanofiber films with superior performance in supercapacitors. Appl. Surf. Sci. 2018, 456, 568–576. [Google Scholar] [CrossRef]
- Seo, D.K.; Jeun, J.P.; Kim, H.B.; Kang, P.H. Preparation and characterization of the carbon nanofiber mat produced from electrospun pan/lignin precursors by electron beam irradiation. Rev. Adv. Mater. Sci. 2011, 28, 31–34. [Google Scholar]
- Lai, C.; Kolla, P.; Zhao, Y.; Fong, H.; Smirnova, A.L. Lignin-derived electrospun carbon nanofiber mats with supercritically deposited Ag nanoparticles for oxygen reduction reaction in alkaline fuel cells. Electrochim. Acta 2014, 130, 431–438. [Google Scholar] [CrossRef]
- Lei, D.; Li, X.D.; Seo, M.K.; Khil, M.S.; Kim, H.Y.; Kim, B.S. NiCo2O4 nanostructure-decorated PAN/lignin based carbon nanofiber electrodes with excellent cyclability for flexible hybrid supercapacitors. Polymer (Guildf.) 2017, 132, 31–40. [Google Scholar] [CrossRef]
- Ding, R.; Wu, H.; Thunga, M.; Bowler, N.; Kessler, M.R. Processing and characterization of low-cost electrospun carbon fibers from organosolv lignin/polyacrylonitrile blends. Carbon N. Y. 2016, 100, 126–136. [Google Scholar] [CrossRef]
- Haider, K.; Ullah, A.; Sarwar, M.N.; Saito, Y.; Sun, L.; Park, S.; Kim, I.S. Lignin-mediated in-situ synthesis of CuO nanoparticles on cellulose nanofibers: A potential wound dressing material. Int. J. Biol. Macromol. 2021. [Google Scholar] [CrossRef]
- Saratale, R.G.; Saratale, G.D.; Ghodake, G.; Cho, S.K.; Kadam, A.; Kumar, G.; Jeon, B.H.; Pant, D.; Bhatnagar, A.; Shin, H.S. Wheat straw extracted lignin in silver nanoparticles synthesis: Expanding its prophecy towards antineoplastic potency and hydrogen peroxide sensing ability. Int. J. Biol. Macromol. 2019, 128, 391–400. [Google Scholar] [CrossRef]
- Aadil, K.R.; Barapatre, A.; Meena, A.S.; Jha, H. Hydrogen peroxide sensing and cytotoxicity activity of Acacia lignin stabilized silver nanoparticles. Int. J. Biol. Macromol. 2016, 82, 39–47. [Google Scholar] [CrossRef]
- Hu, S.; Hsieh, Y. Lo Silver nanoparticle synthesis using lignin as reducing and capping agents: A kinetic and mechanistic study. Int. J. Biol. Macromol. 2016, 82, 856–862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marulasiddeshwara, M.B.; Dakshayani, S.S.; Sharath Kumar, M.N.; Chethana, R.; Raghavendra Kumar, P.; Devaraja, S. Facile-one pot-green synthesis, antibacterial, antifungal, antioxidant and antiplatelet activities of lignin capped silver nanoparticles: A promising therapeutic agent. Mater. Sci. Eng. C 2017, 81, 182–190. [Google Scholar] [CrossRef]
- Xue, Y.; Qiu, X.; Liu, Z.; Li, Y. Facile and Efficient Synthesis of Silver Nanoparticles Based on Biorefinery Wood Lignin and Its Application as the Optical Sensor. ACS Sustain. Chem. Eng. 2018, 6, 7695–7703. [Google Scholar] [CrossRef]
- LIN, X.; Wang, J.; Han, X.; Wu, M.; Kuga, S.; Huang, Y. Use of Lignin and Hemicelluloses for Facial Synthesis of Gold, Platinum, and Palladium Nanoparticles. J. Bioresour. Bioprod. 2017, 2, 149–152. [Google Scholar] [CrossRef]
- Hu, S.; Hsieh, Y. Synthesis of surface bound silver nanoparticles on cellulose fibers using lignin as multi-functional agent. Carbohydr. Polym. 2015, 131, 134–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, L.; Huang, S.; Zheng, J.; Qiu, Z.; Lin, X.; Qin, Y. Synthesis and characterization of biomass lignin-based PVA super-absorbent hydrogel. Int. J. Biol. Macromol. 2019, 140, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Ullah, S.; Khan, M.Q.; Hashmi, M.; Nam, P.D.; Kato, Y.; Tamada, Y.; Kim, I.S. Manuka honey incorporated cellulose acetate nanofibrous mats: Fabrication and in vitro evaluation as a potential wound dressing. Int. J. Biol. Macromol. 2020, 155, 479–489. [Google Scholar] [CrossRef]
- Ullah, A.; Saito, Y.; Ullah, S.; Haider, M.K.; Nawaz, H.; Duy-Nam, P.; Kharaghani, D.; Kim, I.S. Bioactive Sambong oil-loaded electrospun cellulose acetate nanofibers: Preparation, characterization, and in-vitro biocompatibility. Int. J. Biol. Macromol. 2020. [Google Scholar] [CrossRef]
- Arshad, S.N.; Naraghi, M.; Chasiotis, I. Strong carbon nanofibers from electrospun polyacrylonitrile. Carbon N. Y. 2011, 49, 1710–1719. [Google Scholar] [CrossRef]
- Kampalanonwat, P.; Supaphol, P. Preparation and adsorption behavior of aminated electrospun polyacrylonitrile nanofiber mats for heavy metal ion removal. ACS Appl. Mater. Interfaces 2010, 2, 3619–3627. [Google Scholar] [CrossRef]
- Cipriani, E.; Zanetti, M.; Bracco, P.; Brunella, V.; Luda, M.P.; Costa, L. Crosslinking and carbonization processes in PAN films and nanofibers. Polym. Degrad. Stab. 2016, 123, 178–188. [Google Scholar] [CrossRef]
- Wu, S.H.; Qin, X.H. Effects of the stabilization temperature on the structure and properties of polyacrylonitrile-based stabilized electrospun nanofiber microyarns. J. Therm. Anal. Calorim. 2014, 116, 303–308. [Google Scholar] [CrossRef]
- Wangxi, Z.; Jie, L.; Gang, W. Evolution of structure and properties of PAN precursors during their conversion to carbon fibers. Carbon N. Y. 2003, 41, 2805–2812. [Google Scholar] [CrossRef]
- Dalton, S.; Heatley, F.; Budd, P.M. Thermal stabilization of polyacrylonitrile fibres. Polymer (Guildf.) 1999, 40, 5531–5543. [Google Scholar] [CrossRef]
- Frank, E.; Steudle, L.M.; Ingildeev, D.; Spörl, J.M.; Buchmeiser, M.R. Carbon fibers: Precursor systems, processing, structure, and properties. Angew. Chemie Int. Ed. 2014, 53, 5262–5298. [Google Scholar] [CrossRef]
- Viana, R.B.; Da Silva, A.B.F.; Pimentel, A.S. Infrared spectroscopy of anionic, cationic, and zwitterionic surfactants. Adv. Phys. Chem. 2012, 2012. [Google Scholar] [CrossRef] [Green Version]
- Zussman, E.; Chen, X.; Ding, W.; Calabri, L.; Dikin, D.A.; Quintana, J.P.; Ruoff, R.S. Mechanical and structural characterization of electrospun PAN-derived carbon nanofibers. Carbon N. Y. 2005, 43, 2175–2185. [Google Scholar] [CrossRef]
- Bao, Y.; Chen, K. AgCl/Ag/g-C3N4 Hybrid Composites: Preparation, Visible Light-Driven Photocatalytic Activity and Mechanism. Nano-Micro Lett. 2016, 8, 182–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Kasraei, S.; Azarsina, M. Addition of silver nanoparticles reduces the wettability of methacrylate and silorane-based composites. Braz. Oral Res. 2012, 26, 505–510. [Google Scholar] [CrossRef]
- Januariyasa, I.K.; Ana, I.D.; Yusuf, Y. Nanofibrous poly (vinyl alcohol)/chitosan contained carbonated hydroxyapatite nanoparticles scaffold for bone tissue engineering. Mater. Sci. Eng. C 2020, 107, 110347. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.X.; Wang, Y.Z.; Sun, C.F. Characterization on oxidative stabilization of polyacrylonitrile nanofibers prepared by electrospinning. J. Polym. Res. 2007, 14, 467–474. [Google Scholar] [CrossRef]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramírez, J.T.; Yacaman, M.J. The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 16, 2346–2353. [Google Scholar] [CrossRef] [Green Version]
- Selvaraj, S.; Thangam, R.; Fathima, N.N. Electrospinning of casein nanofibers with silver nanoparticles for potential biomedical applications. Int. J. Biol. Macromol. 2018, 120, 1674–1681. [Google Scholar] [CrossRef]
- Feng, Q.L.; Wu, J.; Chen, G.Q.; Cui, F.Z.; Kim, T.N.; Kim, J.O. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J. Biomed. Mater. Res. 2000, 52, 662–668. [Google Scholar] [CrossRef]
- Srivastava, C.M.; Purwar, R.; Gupta, A.P. Enhanced potential of biomimetic, silver nanoparticles functionalized Antheraea mylitta (tasar) silk fibroin nanofibrous mats for skin tissue engineering. Int. J. Biol. Macromol. 2019, 130, 437–453. [Google Scholar] [CrossRef]
- Rehan, M.; Nada, A.A.; Khattab, T.A.; Abdelwahed, N.A.M.; El-Kheir, A.A.A. Development of multifunctional polyacrylonitrile/silver nanocomposite films: Antimicrobial activity, catalytic activity, electrical conductivity, UV protection and SERS-active sensor. J. Mater. Res. Technol. 2020, 9, 9380–9394. [Google Scholar] [CrossRef]
- Dizhbite, T.; Telysheva, G.; Jurkjane, V.; Viesturs, U. Characterization of the radical scavenging activity of lignins—Natural antioxidants. Bioresour. Technol. 2004, 95, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Ugartondo, V.; Mitjans, M.; Vinardell, M.P. Comparative antioxidant and cytotoxic effects of lignins from different sources. Bioresour. Technol. 2008, 99, 6683–6687. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Robles, J.; Larrañeta, E.; Fong, M.L.; Martin, N.K.; Irwin, N.J.; Mutjé, P.; Tarrés, Q.; Delgado-Aguilar, M. Lignin/poly (butylene succinate) composites with antioxidant and antibacterial properties for potential biomedical applications. Int. J. Biol. Macromol. 2020, 145, 92–99. [Google Scholar] [CrossRef]











| AgNO3 Concentration | Alkali Lignin Amount | Sample Code |
|---|---|---|
| 5 mM | 2 g/L | PAN/AgNPs1 |
| 4 g/L | PAN/AgNPs2 | |
| 10mM | 2 g/L | PAN/AgNPs3 |
| 4 g/L | PAN/AgNPs4 | |
| 15 mM | 2 g/L | PAN/AgNPs5 |
| 4 g/L | PAN/AgNPs6 |
| Sample Code | Thickness of the Membrane (mm) | Average Diameter of Nanofibers (nm) | Average Diameter of AgNPs (nm) |
|---|---|---|---|
| PAN nanofiber | 0.014 | 512 ± 73 | ‒ ‒ ‒ ‒ |
| PAN/AgNPs1 | 0.06 | 604 ± 39 | 9 ± 2.3 |
| PAN/AgNPs2 | 0.06 | 614 ± 46 | 14 ± 3.0 |
| PAN/AgNPs3 | 0.04 | 605 ± 59 | 14 ± 2.6 |
| PAN/AgNPs4 | 0.02 | 642 ± 82 | 15 ± 3.9 |
| PAN/AgNPs5 | 0.13 | 665 ± 86 | 16 ± 4.2 |
| PAN/AgNPs6 | 0.05 | 673 ± 98 | 12 ± 2.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haider, M.K.; Ullah, A.; Sarwar, M.N.; Yamaguchi, T.; Wang, Q.; Ullah, S.; Park, S.; Kim, I.S. Fabricating Antibacterial and Antioxidant Electrospun Hydrophilic Polyacrylonitrile Nanofibers Loaded with AgNPs by Lignin-Induced In-Situ Method. Polymers 2021, 13, 748. https://doi.org/10.3390/polym13050748
Haider MK, Ullah A, Sarwar MN, Yamaguchi T, Wang Q, Ullah S, Park S, Kim IS. Fabricating Antibacterial and Antioxidant Electrospun Hydrophilic Polyacrylonitrile Nanofibers Loaded with AgNPs by Lignin-Induced In-Situ Method. Polymers. 2021; 13(5):748. https://doi.org/10.3390/polym13050748
Chicago/Turabian StyleHaider, Md. Kaiser, Azeem Ullah, Muhammad Nauman Sarwar, Takumi Yamaguchi, Qianyu Wang, Sana Ullah, Soyoung Park, and Ick Soo Kim. 2021. "Fabricating Antibacterial and Antioxidant Electrospun Hydrophilic Polyacrylonitrile Nanofibers Loaded with AgNPs by Lignin-Induced In-Situ Method" Polymers 13, no. 5: 748. https://doi.org/10.3390/polym13050748
APA StyleHaider, M. K., Ullah, A., Sarwar, M. N., Yamaguchi, T., Wang, Q., Ullah, S., Park, S., & Kim, I. S. (2021). Fabricating Antibacterial and Antioxidant Electrospun Hydrophilic Polyacrylonitrile Nanofibers Loaded with AgNPs by Lignin-Induced In-Situ Method. Polymers, 13(5), 748. https://doi.org/10.3390/polym13050748