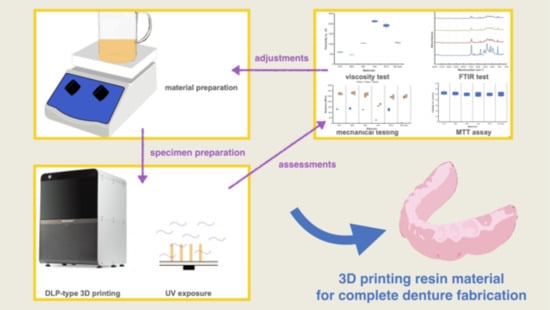
Mechanical Properties and Biocompatibility of Urethane Acrylate-Based 3D-Printed Denture Base Resin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Formulation of UA-Based Photopolymer Resins
2.3. Specimen Fabrication
2.4. Characterization
2.5. Statistical Analysis
3. Results and Discussions
3.1. Viscosity
3.2. FTIR Spectrum and Calculation of Degree of Conversion
3.3. Mechanical Properties
3.4. Cytotoxicity Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muhammad, S.Z. Prosthodontic Applications of Polymethyl Methacrylate (PMMA): An Update. Polymers 2020, 12, 2299. [Google Scholar]
- AlHelal, A.; AlRumaih, H.S.; Kattadiyil, M.T.; Baba, N.Z.; Goodacre, C.J. Comparison of retention between maxillary milled and conventional denture bases: A clinical study. J. Prosthet. Dent. 2017, 117, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Goodacre, B.J.; Goodacre, C.J.; Baba, N.Z.; Kattadiyil, M.T. Comparison of denture base adaptation between CAD-CAM and conventional fabrication techniques. J. Prosthet. Dent. 2016, 116, 249–256. [Google Scholar] [CrossRef]
- Sayed, M.E.; Porwal, A.; Ehrenberg, D.; Weiner, S. Effect of cast modification on denture base adaptation following maxillary complete denture processing. J. Prosthodont. 2019, 28, e6–e12. [Google Scholar] [CrossRef] [Green Version]
- Kalberer, N.; Mehl, A.; Schimmel, M.; Müller, F.; Srinivasan, M. CAD-CAM milled versus rapidly prototyped (3D-printed) complete dentures: An in vitro evaluation of trueness. J. Prosthet. Dent. 2019, 121, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Wimmer, T.; Gallus, K.; Eichberger, M.; Stawarczyk, B. Complete denture fabrication supported by CAD/CAM. J. Prosthet. Dent. 2016, 115, 541–546. [Google Scholar] [CrossRef]
- Fernandez, P.K.; Unkovskiy, A.; Benkendorff, V.; Klink, A.; Spintzyk, S. Surface characteristics of milled and 3D printed denture base materials following polishing and coating: An in-vitro study. Materials 2020, 13, 3305. [Google Scholar] [CrossRef] [PubMed]
- Barkane, A.; Platnieks, O.; Jurinovs, M.; Gaidukovs, S. Thermal stability of UV-cured vegetable oil epoxidized acrylate-based polymer system for 3D printing application. Polym. Degrad. Stab. 2020, 181, 109347. [Google Scholar] [CrossRef]
- Han, H.; Cho, S. Fabrication of conducting polyacrylate resin solution with polyaniline nanofiber and graphene for conductive 3D printing application. Polymers 2018, 10, 1003. [Google Scholar] [CrossRef] [Green Version]
- Hwang, H.-J.; Lee, S.J.; Park, E.-J.; Yoon, H.-I. Assessment of the trueness and tissue surface adaptation of CAD-CAM maxillary denture bases manufactured using digital light processing. J. Prosthet. Dent. 2019, 121, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Sa, L.; Kaiwu, L.; Shenggui, C.; Junzhong, Y.; Yongguang, J.; Lin, W.; Li, R. 3D printing dental composite resins with sustaining antibacterial ability. J. Mater. Sci. 2019, 54, 3309–3318. [Google Scholar] [CrossRef]
- Lebedevaite, M.; Ostrauskaite, J.; Skliutas, E.; Malinauskas, M. Photoinitiator free resins composed of plant-derived monomers for the optical µ-3D printing of thermosets. Polymers 2019, 11, 116. [Google Scholar] [CrossRef] [Green Version]
- Chiu, Y.-C.; Shen, Y.-F.; Lee, A.K.-X.; Lin, S.-H.; Wu, Y.-C.; Chen, Y.-W. 3D printing of amino resin-based photosensitive materials on multi-parameter optimization design for vascular engineering applications. Polymers 2019, 11, 1394. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.-G.; Yang, J.; Jia, Y.-G.; Lu, B.; Ren, L. TiO2 and PEEK reinforced 3D printing PMMA composite resin for dental denture base applications. Nanomaterials 2019, 9, 1049. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.-H.; Lin, Y.-M.; Lai, Y.-L.; Lee, S.-Y. Mechanical properties, accuracy, and cytotoxicity of UV-polymerized 3D printing resins composed of Bis-EMA, UDMA, and TEGDMA. J. Prosthet. Dent. 2020, 123, 349–354. [Google Scholar] [CrossRef]
- Chen, H.; Lee, S.-Y.; Lin, Y.-M. Synthesis and formulation of PCL-based urethane acrylates for DLP 3D printers. Polymers 2020, 12, 1500. [Google Scholar] [CrossRef]
- Kuhnt, T.; García, R.M.; Camarero-Espinosa, S.; Dias, A.; Ten Cate, A.T.; van Blitterswijk, C.A.; Moroni, L.; Baker, M.B. Poly (caprolactone-co-trimethylenecarbonate) urethane acrylate resins for digital light processing of bioresorbable tissue engineering implants. Biomater. Sci. 2019, 7, 4984–4989. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.; Li, J.; He, Z.; Hong, J.; Bao, J. Urethane acrylate-based photosensitive resin for three-dimensional printing of stereolithographic elastomer. J. Appl. Polym. Sci. 2020, 137, 49294. [Google Scholar] [CrossRef]
- Dizon, J.R.C.; Espera, A.H., Jr.; Chen, Q.; Advincula, R.C. Mechanical characterization of 3D-printed polymers. Addit. Manuf. 2018, 20, 44–67. [Google Scholar] [CrossRef]
- Molinero-Mourelle, P.; Canals, S.; Gómez-Polo, M.; Fernanda Solá-Ruiz, M.; del Río Highsmith, J.; Celemín Viñuela, A.; Solá-Ruiz, M.F.; Viñuela, A.C. Polylactic acid as a material for three-dimensional printing of provisional restorations. Int. J Prosthodont. 2018, 31, 349–350. [Google Scholar] [CrossRef]
- Pongprueksa, P.; De Munck, J.; Duca, R.C.; Poels, K.; Covaci, A.; Hoet, P.; Godderis, L.; Van Meerbeek, B.; Van Landuyt, K.L. Monomer elution in relation to degree of conversion for different types of composite. J. Dent. 2015, 43, 1448–1455. [Google Scholar] [CrossRef]
- Ligon, S.C.; Husar, B.; Wutzel, H.; Holman, R.; Liska, R. Strategies to reduce oxygen inhibition in photoinduced polymerization. Chem. Rev. 2014, 114, 557–589. [Google Scholar] [CrossRef] [PubMed]
- Shan, J.; Yang, Z.; Chen, G.; Hu, Y.; Luo, Y.; Dong, X.; Zheng, W.; Zhou, W. Design and synthesis of free-radical/cationic photosensitive resin applied for 3D printer with liquid crystal display (LCD) irradiation. Polymers 2020, 12, 1346. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.-H.; Choi, J.-H.; Kim, H.-J. Coating performance and characteristics for UV-curable aliphatic urethane acrylate coatings containing norrish type I photoinitiators. J. Coat. Technol. Res. 2006, 3, 221–229. [Google Scholar] [CrossRef]
- Ping, T.; Zhou, Y.; He, Y.; Tang, Y.; Yang, J.; Akram, M.Y.; Nie, J. Preparation and characterization of yellowing resistance and low volume shrinkage of fluorinated polysiloxane urethane acrylate. Prog. Org. Coat. 2016, 97, 74–81. [Google Scholar] [CrossRef]
- Mondschein, R.J.; Kanitkar, A.; Williams, C.B.; Verbridge, S.S.; Long, T.E. Polymer structure-property requirements for stereolithographic 3D printing of soft tissue engineering scaffolds. Biomaterials 2017, 140, 170–188. [Google Scholar] [CrossRef] [PubMed]







| Group Name | Monomer | ||||
|---|---|---|---|---|---|
| UA | PET5EO4A | TPO | IBOA | Acrylic | |
| 87A | 40% aliphatic urethane hexa-acrylate | 40 | 3 | 12 | 5 |
| 88A | 40% aromatic urethane hexa-acrylate | 40 | 3 | 12 | 5 |
| 588 | 40% aliphatic urethane acrylate | 40 | 3 | 12 | 5 |
| 594 | 40% aliphatic urethane triacrylate diluted in 15% HDDA | 40 | 3 | 12 | 5 |
| 5812 | 40% high functional aliphatic urethane acrylate | 40 | 3 | 12 | 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tzeng, J.-J.; Yang, T.-S.; Lee, W.-F.; Chen, H.; Chang, H.-M. Mechanical Properties and Biocompatibility of Urethane Acrylate-Based 3D-Printed Denture Base Resin. Polymers 2021, 13, 822. https://doi.org/10.3390/polym13050822
Tzeng J-J, Yang T-S, Lee W-F, Chen H, Chang H-M. Mechanical Properties and Biocompatibility of Urethane Acrylate-Based 3D-Printed Denture Base Resin. Polymers. 2021; 13(5):822. https://doi.org/10.3390/polym13050822
Chicago/Turabian StyleTzeng, Jy-Jiunn, Tzu-Sen Yang, Wei-Fang Lee, Hsuan Chen, and Hung-Ming Chang. 2021. "Mechanical Properties and Biocompatibility of Urethane Acrylate-Based 3D-Printed Denture Base Resin" Polymers 13, no. 5: 822. https://doi.org/10.3390/polym13050822
APA StyleTzeng, J. -J., Yang, T. -S., Lee, W. -F., Chen, H., & Chang, H. -M. (2021). Mechanical Properties and Biocompatibility of Urethane Acrylate-Based 3D-Printed Denture Base Resin. Polymers, 13(5), 822. https://doi.org/10.3390/polym13050822