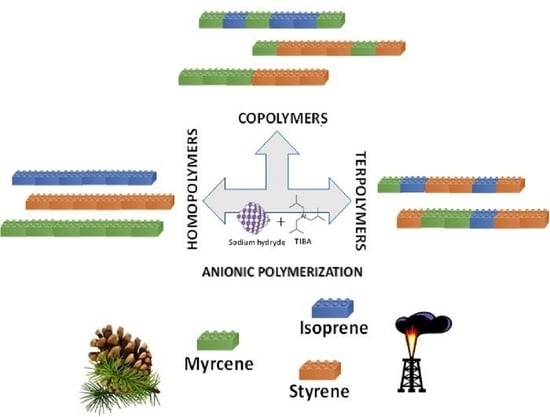
Sustainable Myrcene-Based Elastomers via a Convenient Anionic Polymerization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of NaH/i-Bu3Al Initiator System
2.3. Typical Polymerization Procedure
2.4. Typical Co-polymerization Procedure
2.5. Preparation of Diblock Copolymer
2.6. Polymer Characterizations
3. Results and Discussion
3.1. Homopolymerizations of Myrcene, Styrene and Isoprene
3.2. Synthesis and Characterization of Poly(M−co-S), Poly(M−b-S), Poly(M−co-I) Copolymers and Poly(M-co-S-co-I) Terpolymers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Plastics Europe. Available online: https://www.plasticseurope.org/en (accessed on 24 March 2020).
- Llevot, A.; Meier, M.A.R. Renewability—A Principle of Utmost Importance! Green Chem. 2016, 18, 4800–4803. [Google Scholar] [CrossRef]
- Mathers, R.T.; Meier, M.A.R. Green Polymerization Methods: Renewable Starting Materials, Catalysis and Waste Reduction; Wiley: Hoboken, NJ, USA, 2011; ISBN 978-3-527-63617-4. [Google Scholar]
- Kristufek, S.L.; Wacker, K.T.; Tsao, Y.-Y.T.; Su, L.; Wooley, K.L. Monomer Design Strategies to Create Natural Product-Based Polymer Materials. Nat. Prod. Rep. 2017, 34, 433–459. [Google Scholar] [CrossRef]
- Gandini, A. Polymers from Renewable Resources: A Challenge for the Future of Macromolecular Materials. Macromolecules 2008, 41, 9491–9504. [Google Scholar] [CrossRef]
- Meier, M.A.R.; Metzger, J.O.; Schubert, U.S. Plant Oil Renewable Resources as Green Alternatives in Polymer Science. Chem. Soc. Rev. 2007, 36, 1788–1802. [Google Scholar] [CrossRef]
- Della Monica, F.; Kleij, A.W. From Terpenes to Sustainable and Functional Polymers. Polym. Chem. 2020. [Google Scholar] [CrossRef]
- Winnacker, M. Pinenes: Abundant and Renewable Building Blocks for a Variety of Sustainable Polymers. Angew. Chem. Int. Ed. 2018, 57, 14362–14371. [Google Scholar] [CrossRef] [PubMed]
- Winnacker, M.; Neumeier, M.; Zhang, X.; Papadakis, C.M.; Rieger, B. Sustainable Chiral Polyamides with High Melting Temperature via Enhanced Anionic Polymerization of a Menthone-Derived Lactam. Macromol. Rapid Commun. 2016, 37, 851–857. [Google Scholar] [CrossRef]
- Winnacker, M.; Lamparelli, D.H.; Capacchione, C.; Güngör, H.H.; Stieglitz, L.; Rodewald, K.S.; Schmidt, M.; Gronauer, T.F. Sustainable Polyesteramides and Copolyamides: Insights into the Copolymerization Behavior of Terpene-Based Lactams. Macromol. Chem. Phys. 2020, 221, 2000110. [Google Scholar] [CrossRef]
- Zhao, J.; Schlaad, H. Synthesis of Terpene-Based Polymers. In Bio-Synthetic Polymer Conjugates; Schlaad, H., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; Volume 253, pp. 151–190. ISBN 978-3-642-34349-0. [Google Scholar]
- Breitmaier, E. Terpenes: Flavors, Fragrances, Pharmaca, Pheromones; WILEY-VCH: Weinheim, Germany, 2006; ISBN 978-3-527-31786-8. [Google Scholar]
- Johanson, A.J.; McKennon, F.L.; Goldblatt, L.A. Emulsion Polymerization of Myrcene. Ind. Eng. Chem. 1948, 40, 500–502. [Google Scholar] [CrossRef]
- Runckel, W.J.; Goldblatt, L.A. Inhibition of Myrcene Polymerization during Storage. Ind. Eng. Chem. 1946, 38, 749–751. [Google Scholar] [CrossRef]
- Behr, A.; Johnen, L. Myrcene as a Natural Base Chemical in Sustainable Chemistry: A Critical Review. ChemSusChem 2009, 2, 1072–1095. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.-M.; Eom, J.-H.; Um, Y.; Kim, Y.; Woo, H.M. Microbial Synthesis of Myrcene by Metabolically Engineered Escherichia coli. J. Agric. Food Chem. 2015, 63, 4606–4612. [Google Scholar] [CrossRef]
- Lamparelli, D.H.; Paradiso, V.; Monica, F.D.; Proto, A.; Guerra, S.; Giannini, L.; Capacchione, C. Toward More Sustainable Elastomers: Stereoselective Copolymerization of Linear Terpenes with Butadiene. Macromolecules 2020. [Google Scholar] [CrossRef]
- Li, W.; Zhao, J.; Zhang, X.; Gong, D. Capability of PN 3 -Type Cobalt Complexes toward Selective (Co-)Polymerization of Myrcene, Butadiene, and Isoprene: Access to Biosourced Polymers. Ind. Eng. Chem. Res. 2019, 58, 2792–2800. [Google Scholar] [CrossRef]
- Lamparelli, D.H.; Paradiso, V.; Capacchione, C. New Elastomeric Materials from Biomass: Stereoselective Polymerization of Linear Terpenes and Their Copolymerization with Butadiene by Using a Cobalt Complex With Phosphane Ligands. Rubber Chem. Technol. 2020, 93, 605–614. [Google Scholar] [CrossRef]
- González-Zapata, J.L.; Enríquez-Medrano, F.J.; López González, H.R.; Revilla-Vázquez, J.; Carrizales, R.M.; Georgouvelas, D.; Valencia, L.; Díaz de León Gómez, R.E. Introducing Random Bio-Terpene Segments to High Cis -Polybutadiene: Making Elastomeric Materials More Sustainable. RSC Adv. 2020, 10, 44096–44102. [Google Scholar] [CrossRef]
- Naddeo, M.; Buonerba, A.; Luciano, E.; Grassi, A.; Proto, A.; Capacchione, C. Stereoselective Polymerization of Biosourced Terpenes β-Myrcene and β-Ocimene and Their Copolymerization with Styrene Promoted by Titanium Catalysts. Polymer 2017, 131, 151–159. [Google Scholar] [CrossRef]
- Laur, E.; Welle, A.; Vantomme, A.; Brusson, J.-M.; Carpentier, J.-F.; Kirillov, E. Stereoselective Copolymerization of Styrene with Terpenes Catalyzed by an Ansa-Lanthanidocene Catalyst: Access to New Syndiotactic Polystyrene-Based Materials. Catalysts 2017, 7, 361. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, P.; Bhowmick, A.K. Terpene Based Sustainable Elastomer for Low Rolling Resistance and Improved Wet Grip Application: Synthesis, Characterization and Properties of Poly(Styrene-Co-Myrcene). ACS Sustain. Chem. Eng. 2016, 4, 5462–5474. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, J.; Su, K.; Wang, D.; Han, B. Bio-based Β-myrcene-modified Solution-polymerized Styrene–Butadiene Rubber for Improving Carbon Black Dispersion and Wet Skid Resistance. J. Appl. Polym. Sci. 2019, 136, 48159. [Google Scholar] [CrossRef]
- Bolton, J.M.; Hillmyer, M.A.; Hoye, T.R. Sustainable Thermoplastic Elastomers from Terpene-Derived Monomers. ACS Macro Lett. 2014, 3, 717–720. [Google Scholar] [CrossRef]
- Ren, X.; Guo, F.; Fu, H.; Song, Y.; Li, Y.; Hou, Z. Scandium-Catalyzed Copolymerization of Myrcene with Ethylene and Propylene: Convenient Syntheses of Versatile Functionalized Polyolefins. Polym. Chem. 2018, 9, 1223–1233. [Google Scholar] [CrossRef]
- Trumbo, D.L. Free Radical Copolymerization Behavior of Myrcene: I. Copolymers with Styrene, Methyl Methacrylate or p-Fluorostyrene. Polym. Bull. 1993, 31, 629–636. [Google Scholar] [CrossRef]
- Sarkar, P.; Bhowmick, A.K. Terpene Based Sustainable Methacrylate Copolymer Series by Emulsion Polymerization: Synthesis and Structure-Property Relationship. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 2639–2649. [Google Scholar] [CrossRef]
- Lei, W.; Yang, X.; Qiao, H.; Shi, D.; Wang, R.; Zhang, L. Renewable Resource-Based Elastomer Nanocomposite Derived from Myrcene, Ethanol, Itaconic Acid and Nanosilica: Design, Preparation and Properties. Eur. Polym. J. 2018, 106, 1–8. [Google Scholar] [CrossRef]
- Zhou, C.; Wei, Z.; Lei, X.; Li, Y. Fully Biobased Thermoplastic Elastomers: Synthesis and Characterization of Poly(l-Lactide)-b-Polymyrcene-b-Poly(l-Lactide) Triblock Copolymers. RSC Adv. 2016, 6, 63508–63514. [Google Scholar] [CrossRef]
- Georges, S.; Touré, A.O.; Visseaux, M.; Zinck, P. Coordinative Chain Transfer Copolymerization and Terpolymerization of Conjugated Dienes. Macromolecules 2014, 47, 4538–4547. [Google Scholar] [CrossRef]
- You, F.; Zhai, J.; So, Y.-M.; Shi, X. Rigid Acridane-Based Pincer Supported Rare-Earth Complexes for Cis-1,4-Polymerization of 1,3-Conjugated Dienes. Inorg. Chem. 2021, 60, 1797–1805. [Google Scholar] [CrossRef] [PubMed]
- Sahu, P.; Sarkar, P.; Bhowmick, A.K. Design of a Molecular Architecture via a Green Route for an Improved Silica Reinforced Nanocomposite Using Bioresources. ACS Sustain. Chem. Eng. 2018, 6, 6599–6611. [Google Scholar] [CrossRef]
- Bauer, N.; Brunke, J.; Kali, G. Controlled Radical Polymerization of Myrcene in Bulk: Mapping the Effect of Conditions on the System. ACS Sustain. Chem. Eng. 2017, 5, 10084–10092. [Google Scholar] [CrossRef]
- Métafiot, A.; Kanawati, Y.; Gérard, J.-F.; Defoort, B.; Marić, M. Synthesis of β-Myrcene-Based Polymers and Styrene Block and Statistical Copolymers by SG1 Nitroxide-Mediated Controlled Radical Polymerization. Macromolecules 2017, 50, 3101–3120. [Google Scholar] [CrossRef]
- Kalita, U.; Samanta, S.; Banerjee, S.L.; Das, N.C.; Singha, N.K. Biobased Thermoplastic Elastomer Based on an SMS Triblock Copolymer Prepared via RAFT Polymerization in Aqueous Medium. Macromolecules 2021, 54, 1478–1488. [Google Scholar] [CrossRef]
- Hulnik, M.I.; Vasilenko, I.V.; Radchenko, A.V.; Peruch, F.; Ganachaud, F.; Kostjuk, S.V. Aqueous Cationic Homo- and Co-Polymerizations of β-Myrcene and Styrene: A Green Route toward Terpene-Based Rubbery Polymers. Polym. Chem. 2018, 9, 5690–5700. [Google Scholar] [CrossRef]
- Quirk, R.P.; Huang, T.-L. Alkyllithium-Initiated Polymerization of Myrcene New Block Copolymers of Styrene and Myrcene. In New Monomers and Polymers; Culbertson, B.M., Pittman, C.U., Eds.; Springer: Boston, MA, USA, 1984; pp. 329–355. ISBN 978-1-4684-4621-0. [Google Scholar]
- Matic, A.; Schlaad, H. Thiol-Ene Photofunctionalization of 1,4-Polymyrcene: Thiol-Ene Photofunctionalization of 1,4-Polymyrcene. Polym. Int. 2018, 67, 500–505. [Google Scholar] [CrossRef]
- Wahlen, C.; Blankenburg, J.; von Tiedemann, P.; Ewald, J.; Sajkiewicz, P.; Müller, A.H.E.; Floudas, G.; Frey, H. Tapered Multiblock Copolymers Based on Farnesene and Styrene: Impact of Biobased Polydiene Architectures on Material Properties. Macromolecules 2020. [Google Scholar] [CrossRef]
- Bareuther, J.; Plank, M.; Kuttich, B.; Kraus, T.; Frey, H.; Gallei, M. Temperature Variation Enables the Design of Biobased Block Copolymers via One-Step Anionic Copolymerization. Macromol. Rapid Commun. 2020, 2000513. [Google Scholar] [CrossRef]
- Gong, D.; Tang, F.; Xu, Y.; Hu, Z.; Luo, W. Cobalt Catalysed Controlled Copolymerization: An Efficient Approach to Bifunctional Polyisoprene with Enhanced Properties. Polym. Chem. 2021. [Google Scholar] [CrossRef]
- Cawse, J.L.; Stanford, J.L.; Still, R.H. Polymers from Renewable Sources: 5. Myrcene-Based Polyols as Rubber-Toughening Agents in Glassy Polyurethanes. Polymer 1987, 28, 368–374. [Google Scholar] [CrossRef]
- Spontak, R.J.; Patel, N.P. Thermoplastic Elastomers: Fundamentals and Applications. Curr. Opin. Colloid Interface Sci. 2000, 5, 333–340. [Google Scholar] [CrossRef]
- Bhowmick, A.K.; Stephens, H.L. (Eds.) Handbook of Elastomers, 2nd ed.; M. Dekker: New York, NY, USA, 2001; ISBN 978-0-8247-0383-7. [Google Scholar]
- Hou, G.; Tao, W.; Liu, J.; Zhang, X.; Dong, M.; Zhang, L. Effect of the Structural Characteristics of Solution Styrene–Butadiene Rubber on the Properties of Rubber Composites. J. Appl. Polym. Sci. 2018, 135, 45749. [Google Scholar] [CrossRef]
- Lewandowski, L.; Sibbald, M.S.; Johnson, E.; Mallamaci, M.P. New Emulsion SBR Technology: Part I. Raw Polymer Study. Rubber Chem. Technol. 2000, 73, 731–742. [Google Scholar] [CrossRef]
- Obrecht, W.; Lambert, J.-P.; Happ, M.; Oppenheimer-Stix, C.; Dunn, J.; Krüger, R. Rubber, 4. Emulsion Rubbers. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2011; pp. 623–646. ISBN 978-3-527-30673-2. [Google Scholar]
- Brandt, H.-D.; Nentwig, W.; Rooney, N.; LaFlair, R.T.; Wolf, U.U.; Duffy, J.; Puskas, J.E.; Kaszas, G.; Drewitt, M.; Glander, S. Rubber, 5. Solution Rubbers. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2011; pp. 649–677. ISBN 978-3-527-30673-2. [Google Scholar]
- Hadjichristidis, N.; Hirao, A. (Eds.) Anionic Polymerization: Principles, Practice, Strength, Consequences and Applications; Springer Japan: Tokyo, Japan, 2015; ISBN 978-4-431-54185-1. [Google Scholar]
- Brown, H.C.; Schlesinger, H.I.; Sheft, I.; Ritter, D.M. Addition Compounds of Alkali Metal Hydrides. Sodium Trimethoxyborohydride and Related Compounds. J. Am. Chem. Soc. 1953, 75, 192–195. [Google Scholar] [CrossRef]
- Ashby, E.C.; Arnott, R.; Srivastava, S. Reactions of Alkali Metal Hydrides with Magnesium Alkyls. Preparation of MMgR2H and MMg2R4H Compounds. Inorg. Chem. 1975, 14, 2422–2426. [Google Scholar] [CrossRef]
- Kubas, G.J.; Shriver, D.F. Nature of Dialkyl- and Diarylzinc Hydride Complexes. J. Am. Chem. Soc. 1970, 92, 1949–1954. [Google Scholar] [CrossRef]
- Ziegler, K.; Köster, R.; Lehmkuhl, H.; Reinert, K. Metallorganische Verbindungen, XXX Neue Komplexverbindungen der Aluminiumalkyle. Justus Liebigs Ann. Chem. 1960, 629, 33–49. [Google Scholar] [CrossRef]
- Needles, H.L. Sodium Hydride-Initiated Polymerizations of Vinyl Monomers in Aprotic Solvents. J. Polym. Sci. Part A-1 Polym. Chem. 1969, 7, 1437–1445. [Google Scholar] [CrossRef]
- Carlotti, S.; Menoret, S.; Desbois, P.; Nissner, N.; Warzelhan, V.; Deffieux, A. Sodium Hydride/Trialkylaluminum Complexes for the Controlled Anionic Polymerization of Styrene at High Temperature. Macromol. Rapid Commun. 2006, 27, 905–909. [Google Scholar] [CrossRef]
- Carlotti, S.; Desbois, P.; Warzelhan, V.; Deffieux, A. Retarded Anionic Polymerization (RAP) of Styrene and Dienes. Polymer 2009, 50, 3057–3067. [Google Scholar] [CrossRef]
- Carlotti, S.; Ménoret, S.; Barabanova, A.; Desbois, P.; Deffieux, A. Sodium Hydride as a New Initiator for the Retarded Anionic Polymerization (RAP) of Styrene. Polymer 2007, 48, 4322–4327. [Google Scholar] [CrossRef]
- Newmark, R.A.; Majumdar, R.N. 13C-NMR Spectra of Cis-Polymyrcene and Cis-Polyfarnesene. J. Polym. Sci. A Polym. Chem. 1988, 26, 71–77. [Google Scholar] [CrossRef]
- Grune, E.; Bareuther, J.; Blankenburg, J.; Appold, M.; Shaw, L.; Müller, A.H.E.; Floudas, G.; Hutchings, L.R.; Gallei, M.; Frey, H. Towards Bio-Based Tapered Block Copolymers: The Behaviour of Myrcene in the Statistical Anionic Copolymerisation. Polym. Chem. 2019, 10, 1213–1220. [Google Scholar] [CrossRef] [Green Version]
- Glatzel, J.; Noack, S.; Schanzenbach, D.; Schlaad, H. Anionic Polymerization of Dienes in ‘Green’ Solvents. Polym. Int. 2020, 70, 181–184. [Google Scholar] [CrossRef]
- Zhang, S.; Han, L.; Ma, H.; Liu, P.; Shen, H.; Lei, L.; Li, C.; Yang, L.; Li, Y. Investigation on Synthesis and Application Performance of Elastomers with Biogenic Myrcene. Ind. Eng. Chem. Res. 2019, 58, 12845–12853. [Google Scholar] [CrossRef]
- Sarkar, P.; Bhowmick, A.K. Terpene-Based Sustainable Elastomers: Vulcanization and Reinforcement Characteristics. Ind. Eng. Chem. Res. 2018, 57, 5197–5206. [Google Scholar] [CrossRef]
- Ishihara, N.; Seimiya, T.; Kuramoto, M.; Uoi, M. Crystalline Syndiotactic Polystyrene. Macromolecules 1986, 19, 2464–2465. [Google Scholar] [CrossRef]
- Labbé, A.; Carlotti, S.; Shcheglova, L.; Desbois, P.; Deffieux, A. Dienes Polymerization in the Presence of Metal Hydrides and Triethylaluminum. Polymer 2006, 47, 3734–3739. [Google Scholar] [CrossRef]
- Liu, B.; Liu, D.; Li, S.; Sun, G.; Cui, D. High Trans-1,4 (Co)Polymerization of β-Myrcene and Isoprene with an Iminophosphonamide Lanthanum Catalyst. Chin. J. Polym. Sci. 2016, 34, 104–110. [Google Scholar] [CrossRef]
- Liu, B.; Li, L.; Sun, G.; Liu, D.; Li, S.; Cui, D. Isoselective 3,4-(Co)Polymerization of Bio-Renewable Myrcene Using NSN-Ligated Rare-Earth Metal Precursor: An Approach to a New Elastomer. Chem. Commun. 2015, 51, 1039–1041. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Zhang, X.; Gong, D. 1,2 Enriched polymerization of isoprene by cobalt complex carrying aminophosphory fused (PN3) ligand. J. Polym. Sci. Part A: Polym. Chem. 2018, 56, 2286–2293. [Google Scholar] [CrossRef]
- Ni, H.; Wang, X. Surface Wetting Behavior of the Poly(Styrene-b-Isoprene-b-Styrene) Triblock Copolymer with Different Chemical Structures of the Polyisoprene Block Chain. Surf. Sci. 2007, 601, 1560–1565. [Google Scholar] [CrossRef]
- Gan, Q.; Xu, Y.; Huang, W.; Luo, W.; Hu, Z.; Tang, F.; Jia, X.; Gong, D. Utilization of Bio-sourced Myrcene for Efficient Preparation of Highly Cis -1,4 Regular Elastomer via a Neodymium Catalyzed Copolymerization Strategy. Polym. Int. 2020. [Google Scholar] [CrossRef]






| Run [a] | Monomers feed [M,S,I][mol %] | Time [h] | Yield [b] [%] | MW [c] [KDa] | Ð [e] | Tg [d] [°C] | Polymer Composition and Microstructure [%] | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| M [1,4/3,4/1,2] | S | I [1,4[cis]/3,4/1,2] | |||||||||
| 1 | 100,0,0 | 6.0 × 10−3 | 500 | 24 | 58 | 50.2 | 1.7 | −61.8 | 100 [46/52/2] | - | - |
| 2 [e] | 100,0,0 | 6.0 × 10−3 | 500 | 24 | 7 | 264.9 | 1.7 | n.d. | 100 | - | - |
| “ | “ | “ | “ | “ | “ | 7.9 | 1.7 | n.d. | “ | “ | “ |
| 3 | 100,0,0 | 3.0 × 10−3 | 1000 | 72 | 85 | 79.6 | 1.7 | n.d. | 100 [49/48/3] | - | - |
| 4 | 100,0,0 | 2.2 × 10−3 | 1450 | 72 | 40 | 89.3 | 2.0 | −76.5 | 100 [51/47/2] | - | - |
| 5 | 0,100,0 | 6.0 × 10−3 | 500 | 8 | 100 | 151.2 | 1.4 | 105.5 | - | 100 | - |
| 6 | 0,100,0 | 2.2 × 10−3 | 1450 | 8 | 91 | 285.1 | 1.5 | n.d. | - | 100 | - |
| 7 | 0,0,100 | 6.0 × 10−3 | 500 | 12 | 40 | 24.9 | 1.5 | n.d. | - | - | 100 [30[53]/56/14] |
| 8 | 0,0,100 | 6.0 × 10−3 | 500 | 24 | 74 | 33.4 | 1.5 | −11.4 | - | - | 100 [32[52]/55/13] |
| 9 | 0,0,100 | 3.0 × 10−3 | 1000 | 24 | 55 | 59.1 | 1.6 | −14.9 | - | - | 100 [35[53]/54/11] |
| Run [a] | Monomers Feed [M,S,I][mol %] | Time [h] | Yield [b] [%] | MW [c] [KDa] | Ð [e] | Tg [f] [°C] | Polymer Composition and Microstructure [%] | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| M [1,4/3,4/1,2] | S | I | |||||||||
| 10 | 90,10,0 | 6.0 × 10−3 | 500 | 72 | 82 | 48.1 | 1.8 | −47.7 | 87 [48/52] | 13 | - |
| 11 | 90,10,0 | 2.2 × 10−3 | 1450 | 72 | 45 | 97.1 | 1.8 | −52.2 | 89 [52/48] | 11 | - |
| 12 | 70,30,0 | 2.2 × 10−3 | 1450 | 72 | 54 | 142.9 | 2.1 | −42.4 | 65 [55/45] | 35 | - |
| 13 | 50,50,0 | 2.2 × 10−3 | 1450 | 72 | 75 | 151.2 | 2.1 | −4.9 | 38 [49/51] | 62 | - |
| 14 | 30,70,0 | 2.2 × 10−3 | 1450 | 72 | 91 | 159.8 | 1.9 | 21.3 | 17 [56/44] | 83 | - |
| 15 | 70,0,30 | 3.0 × 10−3 | 1000 | 72 | 78 | 71.3 | 1.7 | −46.1 | 55 [59/41] | - | 45 |
| 16 | 50,0,50 | 3.0 × 10−3 | 1000 | 72 | 85 | 66.5 | 1.9 | −37.5 | 31 [58/42] | - | 69 |
| 17 | 30,0,70 | 3.0 × 10−3 | 1000 | 72 | 80 | 62.9 | 1.8 | −33.0 | 19 [53/47] | - | 81 |
| 18 | 33,33,34 | 3.0 × 10−3 | 1000 | 72 | 83 | 98.7 | 2.7 | −8.2 | 17 | 52 | 31 |
| 19 | 50,40,10 | 3.0 × 10−3 | 1000 | 72 | 81 | 145.2 | 2.2 | −5.1 | 33 | 54 | 13 |
| 20 | 10,40,50 | 3.0 × 10−3 | 1000 | 72 | 80 | 90.9 | 1.7 | 11.0 | 8 | 45 | 47 |
| 21 [e] | 50,50,0 | 3.0 × 10−3 | 1000 | 8 + 72 | 88 | 60.2 | 1.8 | 98.9 | 36 [46/54] | 64 | - |
| ’ | ’ | ’ | ’ | ’ | ’ | ’ | ’ | −52.5 | ’ | ’ | ’ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lamparelli, D.H.; Kleybolte, M.M.; Winnacker, M.; Capacchione, C. Sustainable Myrcene-Based Elastomers via a Convenient Anionic Polymerization. Polymers 2021, 13, 838. https://doi.org/10.3390/polym13050838
Lamparelli DH, Kleybolte MM, Winnacker M, Capacchione C. Sustainable Myrcene-Based Elastomers via a Convenient Anionic Polymerization. Polymers. 2021; 13(5):838. https://doi.org/10.3390/polym13050838
Chicago/Turabian StyleLamparelli, David Hermann, Magdalena Maria Kleybolte, Malte Winnacker, and Carmine Capacchione. 2021. "Sustainable Myrcene-Based Elastomers via a Convenient Anionic Polymerization" Polymers 13, no. 5: 838. https://doi.org/10.3390/polym13050838
APA StyleLamparelli, D. H., Kleybolte, M. M., Winnacker, M., & Capacchione, C. (2021). Sustainable Myrcene-Based Elastomers via a Convenient Anionic Polymerization. Polymers, 13(5), 838. https://doi.org/10.3390/polym13050838