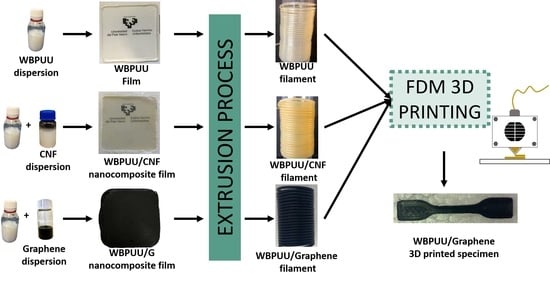
Cellulose and Graphene Based Polyurethane Nanocomposites for FDM 3D Printing: Filament Properties and Printability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of the Waterborne Polyurethane-Urea
2.2. Obtaining of CNF, Carboxylated CNF and Graphene
2.3. Composite Preparation
2.4. Extrusion Process
2.5. Fused Deposition 3D Printing
2.6. Characterization
Biocompatibility Analyses
2.7. Fourier Transform Infrared Spectroscopy (FTIR)
2.8. Thermogravimetric Analysis (TGA)
2.9. Dynamic Mechanical Analysis (DMA)
2.10. Mechanical Testing
2.11. Scanning Electron Microscopy (SEM)
3. Results and Discussion
3.1. Characterization of the Different Nanoreinforcements
3.2. Biocompatibility of WBPUU
3.3. Characterization of Filaments
3.4. Characterization of 3D Printed Parts
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- 3D Printing Trends 2020. Industry Highlights and Market Trends. Available online: https://downloads.3dhubs.com/3D_printing_trends_report_2020.pdf (accessed on 4 February 2021).
- Al-Dulimi, Z.; Wallis, M.; Tan, D.K.; Maniruzzaman, M.; Nokhodchi, A. 3D printing technology as innovative solutions for biomedical applications. Drug Discov. Today 2020. [Google Scholar] [CrossRef]
- Camargo, J.C.; Machado, Á.R.; Almeida, E.C.; Silva, E.F.M.S. Mechanical properties of PLA-graphene filament for FDM 3D printing. Int. J. Adv. Manuf. Technol. 2019, 103, 2423–2443. [Google Scholar] [CrossRef]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.; Hui, D. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Compos. Part B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Chen, Q.; Mangadlao, J.D.; Wallat, J.; De Leon, A.; Pokorski, J.K.; Advincula, R.C. 3D Printing Biocompatible Polyurethane/Poly(lactic acid)/Graphene Oxide Nanocomposites: Anisotropic Properties. ACS Appl. Mater. Interfaces 2017, 9, 4015–4023. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Lv, R.; Bai, S. Recent advances on 3D printing graphene-based composites. Nano Mater. Sci. 2019, 1, 101–115. [Google Scholar] [CrossRef]
- Kim, K.; Park, J.; Suh, J.-H.; Kim, M.; Jeong, Y.; Park, I. 3D printing of multiaxial force sensors using carbon nanotube (CNT)/thermoplastic polyurethane (TPU) filaments. Sens. Actuators A Phys. 2017, 263, 493–500. [Google Scholar] [CrossRef]
- Sadia, M.; Sośnicka, A.; Arafat, B.; Isreb, A.; Ahmed, W.; Kelarakis, A.; Alhnan, M.A. Adaptation of pharmaceutical excipients to FDM 3D printing for the fabrication of patient-tailored immediate release tablets. Int. J. Pharm. 2016, 513, 659–668. [Google Scholar] [CrossRef]
- Christ, J.F.; Hohimer, C.J.; Aliheidari, N.; Ameli, A.; Mo, C.; Pötschke, P. 3D Printing of Highly Elastic Strain Sensors Using Polyurethane/Multiwall Carbon Nanotube Composites. In Sensors and Smart Structures Technologies for Civil, Mechanical, and Aerospace Systems 2017; SPIE: Bellingham, WA, USA, 2017; Volume 101680. [Google Scholar] [CrossRef]
- Przybytek, A.; Gubańska, I.; Kucińska-Lipka, J.; Janik, H. Polyurethanes as a Potential Medical-Grade Filament for Use in Fused Deposition Modeling 3D Printers—A Brief Review. Fibres Text. East. Eur. 2018, 26, 120–125. [Google Scholar] [CrossRef]
- Haryńska, A.; Gubanska, I.; Kucinska-Lipka, J.; Janik, H. Fabrication and Characterization of Flexible Medical-Grade TPU Filament for Fused Deposition Modeling 3DP Technology. Polymers 2018, 10, 1304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santamaria-Echart, A.; Arbelaiz, A.; Saralegi, A.; Fernández-D’Arlas, B.; Eceiza, A.; Corcuera, M.A. Relationship between reagents molar ratio and dispersion stability and film properties of waterborne polyurethanes. Colloids Surfaces A Physicochem. Eng. Asp. 2015, 482, 554–561. [Google Scholar] [CrossRef]
- Korley, L.T.J.; Pate, B.D.; Thomas, E.L.; Hammond, P.T. Effect of the degree of soft and hard segment ordering on the morphology and mechanical behavior of semicrystalline segmented polyurethanes. Polymer 2006, 47, 3073–3082. [Google Scholar] [CrossRef]
- Saralegi, A.; Rueda, L.; Martin, L.; Arbelaiz, A.; Eceiza, A.; Corcuera, M.A. From elastomeric to rigid polyurethane/cellulose nanocrystal bionanocomposites. Compos. Sci. Technol. 2013, 88, 39–47. [Google Scholar] [CrossRef]
- Lu, Y.; LaRock, R.C. Soybean-Oil-Based Waterborne Polyurethane Dispersions: Effects of Polyol Functionality and Hard Segment Content on Properties. Biomacromolecules 2008, 9, 3332–3340. [Google Scholar] [CrossRef]
- Hojabri, L.; Kong, X.; Narine, S.S. Fatty Acid-Derived Diisocyanate and Biobased Polyurethane Produced from Vegetable Oil: Synthesis, Polymerization, and Characterization. Biomacromolecules 2009, 10, 884–891. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, A.; Bose, S.; Das, S. 3D printing of biomaterials. MRS Bull. 2015, 40, 108–115. [Google Scholar] [CrossRef] [Green Version]
- Haryńska, A.; Kucinska-Lipka, J.; Sulowska, A.; Gubanska, I.; Kostrzewa, M.; Janik, H. Medical-Grade PCL Based Polyurethane System for FDM 3D Printing—Characterization and Fabrication. Materials 2019, 12, 887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- all3DP. Available online: https://all3dp.com/2/direct-vs-bowden-extruder-technology-shootout/ (accessed on 2 February 2021).
- Simplify3D. Available online: https://www.simplify3d.com/support/materials-guide/flexible/ (accessed on 2 February 2021).
- Tzounis, L.; Petousis, M.; Grammatikos, S.; Vidakis, N. 3D Printed Thermoelectric Polyurethane/Multiwalled Carbon Nanotube Nanocomposites: A Novel Approach towards the Fabrication of Flexible and Stretchable Organic Thermoelectrics. Materials 2020, 13, 2879. [Google Scholar] [CrossRef] [PubMed]
- Xiang, D.; Zhang, X.; Li, Y.; Harkin-Jones, E.; Zheng, Y.; Wang, L.; Zhao, C.; Wang, P. Enhanced performance of 3D printed highly elastic strain sensors of carbon nanotube/thermoplastic polyurethane nanocomposites via non-covalent interactions. Compos. Part B Eng. 2019, 176. [Google Scholar] [CrossRef]
- Koponen, A.I. The effect of consistency on the shear rheology of aqueous suspensions of cellulose micro- and nanofibrils: A review. Cellulose 2020, 27, 1879–1897. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Ji, C.; Sun, L.; Sun, J.; Liu, J. Cellulose Nanofibrils Filled Poly(Lactic Acid) Biocomposite Filament for FDM 3D Printing. Molecules 2020, 25, 2319. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Lei, Q.; Xing, S. Mechanical characteristics of wood, ceramic, metal and carbon fiber-based PLA composites fabricated by FDM. J. Mater. Res. Technol. 2019, 8, 3741–3751. [Google Scholar] [CrossRef]
- Rastin, H.; Zhang, B.; Mazinani, A.; Hassan, K.; Bi, J.; Tung, T.T.; Losic, D. 3D bioprinting of cell-laden electroconductive MXene nanocomposite bioinks. Nanoscale 2020, 12, 16069–16080. [Google Scholar] [CrossRef]
- Rastin, H.; Zhang, B.; Bi, J.; Hassan, K.; Tung, T.T.; Losic, D. 3D printing of cell-laden electroconductive bioinks for tissue engineering applications. J. Mater. Chem. B 2020, 8, 5862–5876. [Google Scholar] [CrossRef]
- Zhu, X.; Ng, L.W.T.; Hu, G.; Wu, T.; Um, D.; Macadam, N.; Hasan, T. Hexagonal Boron Nitride–Enhanced Optically Transparent Polymer Dielectric Inks for Printable Electronics. Adv. Funct. Mater. 2020, 30, 2002339. [Google Scholar] [CrossRef] [PubMed]
- Santamaria-Echart, A.; Ugarte, L.; García-Astrain, C.; Arbelaiz, A.; Corcuera, M.A.; Eceiza, A. Cellulose nanocrystals reinforced environmentally-friendly waterborne polyurethane nanocomposites. Carbohydr. Polym. 2016, 151, 1203–1209. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ando, M.; Kubota, M.; Ishihara, S.; Hikima, Y.; Ohshima, M.; Sekiguchi, T.; Sato, A.; Yano, H. Effects of hydrophobic-modified cellulose nanofibers (CNFs) on cell morphology and mechanical properties of high void fraction polypropylene nanocomposite foams. Compos. Part A Appl. Sci. Manuf. 2017, 98, 166–173. [Google Scholar] [CrossRef]
- Fallon, J.J.; Kolb, B.Q.; Herwig, C.J.; Foster, E.J.; Bortner, M.J. Mechanically adaptive thermoplastic polyurethane/cellulose nanocrystal composites: Process-driven structure-property relationships. J. Appl. Polym. Sci. 2019, 136, 1–8. [Google Scholar] [CrossRef]
- Giri, B.R.; Poudel, S.; Kim, D.W. Cellulose and its derivatives for application in 3D printing of pharmaceuticals. J. Pharm. Investig. 2021, 51, 1–22. [Google Scholar] [CrossRef]
- Hoeng, F.; Denneulin, A.; Bras, J. Use of nanocellulose in printed electronics: A review. Nanoscale 2016, 8, 13131–13154. [Google Scholar] [CrossRef] [PubMed]
- Rastin, H.; Ormsby, R.T.; Atkins, G.J.; Losic, D. 3D Bioprinting of Methylcellulose/Gelatin-Methacryloyl (MC/GelMA) Bioink with High Shape Integrity. ACS Appl. Bio Mater. 2020, 3, 1815–1826. [Google Scholar] [CrossRef]
- Shi, K.; Salvage, J.P.; Maniruzzaman, M.; Nokhodchi, A. Role of release modifiers to modulate drug release from fused deposition modelling (FDM) 3D printed tablets. Int. J. Pharm. 2021, 597, 120315. [Google Scholar] [CrossRef]
- Stenvall, E.; Flodberg, G.; Pettersson, H.; Hellberg, K.; Hermansson, L.; Wallin, M.; Yang, L. Additive Manufacturing of Prostheses Using Forest-Based Composites. Bioengineering 2020, 7, 103. [Google Scholar] [CrossRef]
- Giubilini, A.; Siqueira, G.; Clemens, F.J.; Sciancalepore, C.; Messori, M.; Nyström, G.; Bondioli, F. 3D-Printing Nanocellulose-Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) Biodegradable Composites by Fused Deposition Modeling. ACS Sustain. Chem. Eng. 2020, 8, 10292–10302. [Google Scholar] [CrossRef]
- Ma, T.; Lv, L.; Ouyang, C.; Hu, X.; Liao, X.; Song, Y.; Hu, X. Rheological behavior and particle alignment of cellulose nanocrystal and its composite hydrogels during 3D printing. Carbohydr. Polym. 2021, 253, 117217. [Google Scholar] [CrossRef]
- Kausar, A. Emerging Research Trends in Polyurethane/Graphene Nanocomposite: A Review. Polym. Plast. Technol. Eng. 2017, 56, 1468–1486. [Google Scholar] [CrossRef]
- Li, C.; Hui, B.; Ye, L. Construction of Polyurethane-imide/Graphene Oxide Nanocomposite Foam with Gradient Structure and Its Thermal Mechanical Stability. Ind. Eng. Chem. Res. 2018, 57, 13742–13752. [Google Scholar] [CrossRef]
- Maria, C.; Lucia, M.; Daniele, M.; Gerardo, C. Graphene in neurosurgery: The beginning of a new era. Nanomedicine 2015, 10, 615–625. [Google Scholar]
- He, H.; Cheng, M.; Liang, Y.; Zhu, H.; Sun, Y.; Dong, D.; Wang, S. Intelligent Cellulose Nanofibers with Excellent Biocompatibility Enable Sustained Antibacterial and Drug Release via a pH-Responsive Mechanism. J. Agric. Food Chem. 2020, 68, 3518–3527. [Google Scholar] [CrossRef] [PubMed]
- Tejado, A.; Alam, N.; Antal, M.; Yang, H.; Van De Ven, T.G.M. Energy requirements for the disintegration of cellulose fibers into cellulose nanofibers. Cellulose 2012, 19, 831–842. [Google Scholar] [CrossRef]
- Larraza, I.; Vadillo, J.; Santamaria-Echart, A.; Tejado, A.; Azpeitia, M.; Vesga, E.; Orue, A.; Saralegi, A.; Arbelaiz, A.; Eceiza, A. The effect of the carboxylation degree on cellulose nanofibers and waterborne polyurethane/cellulose nanofiber nanocomposites properties. Polym. Degrad. Stab. 2020, 173, 109084. [Google Scholar] [CrossRef]
- Larraza, I.; Alonso-Lerma, B.; Gonzalez, K.; Gabilondo, N.; Perez-Jimenez, R.; Corcuera, M.A.; Arbelaiz, A.; Eceiza, A. Waterborne polyurethane and graphene/graphene oxide-based nanocomposites: Reinforcement and electrical conductivity. Express Polym. Lett. 2020, 14, 1018–1033. [Google Scholar] [CrossRef]
- González, K.; García-Astrain, C.; Santamaria-Echart, A.; Ugarte, L.; Avérous, L.; Eceiza, A.; Gabilondo, N. Starch/graphene hydrogels via click chemistry with relevant electrical and antibacterial properties. Carbohydr. Polym. 2018, 202, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Sain, M.; Panthapulakkal, S. Bioprocess preparation of wheat straw fibers and their characterization. Ind. Crops Prod. 2006, 23, 1–8. [Google Scholar] [CrossRef]
- Široký, J.; Blackburn, R.S.; Bechtold, T.; Taylor, J.; White, P. Attenuated total reflectance Fourier-transform Infrared spectroscopy analysis of crystallinity changes in lyocell following continuous treatment with sodium hydroxide. Cellulose 2010, 17, 103–115. [Google Scholar] [CrossRef]
- Jonoobi, M.; Harun, J.; Shakeri, A.; Misra, M.; Oksmand, K. Chemical composition, crystallinity, and thermal degradation of bleached and unbleached kenaf bast (Hibiscus cannabinus) pulp and nanofibers. BioResources 2009, 4, 626–639. [Google Scholar]
- Zhbankov, R.G. Infrared Spectra of Cellulose and Its Derivatives; Springer: Boston, MA, USA, 1995. [Google Scholar]
- Goncalves, G.; Marques, P.A.A.P.; Granadeiro, C.M.; Nogueira, H.I.S.; Singh, M.K.; Grácio, J. Surface Modification of Graphene Nanosheets with Gold Nanoparticles: The Role of Oxygen Moieties at Graphene Surface on Gold Nucleation and Growth. Chem. Mater. 2009, 21, 4796–4802. [Google Scholar] [CrossRef]
- Nethravathi, C.; Rajamathi, M. Chemically modified graphene sheets produced by the solvothermal reduction of colloidal dispersions of graphite oxide. Carbon 2008, 46, 1994–1998. [Google Scholar] [CrossRef]
- Ghobadi, M.; Gharabaghi, M.; Abdollahi, H.; Boroumand, Z.; Moradian, M. MnFe2O4-graphene oxide magnetic nanoparticles as a high-performance adsorbent for rare earth elements: Synthesis, isotherms, kinetics, thermodynamics and desorption. J. Hazard. Mater. 2018, 351, 308–316. [Google Scholar] [CrossRef]
- Santamaria-Echart, A.; Fernandes, I.; Barreiro, F.; Retegi, A.; Arbelaiz, A.; Corcuera, M.A.; Eceiza, A. Development of waterborne polyurethane-ureas added with plant extracts: Study of different incorporation routes and their influence on particle size, thermal, mechanical and antibacterial properties. Prog. Org. Coat. 2018, 117, 76–90. [Google Scholar] [CrossRef] [Green Version]
- Dehghanzad, B.; Aghjeh, M.K.R.; Rafeie, O.; Tavakoli, A.; Oskooie, A.J. Synthesis and characterization of graphene and functionalized graphene via chemical and thermal treatment methods. RSC Adv. 2016, 6, 3578–3585. [Google Scholar] [CrossRef]
- Yue, Y.; Han, G.; Wu, Q. Transitional Properties of Cotton Fibers from Cellulose I to Cellulose II Structure. BioResources 2013, 8, 6460–6471. [Google Scholar] [CrossRef] [Green Version]
- Soni, B.; Hassan, E.B.; Mahmoud, B. Chemical isolation and characterization of different cellulose nanofibers from cotton stalks. Carbohydr. Polym. 2015, 134, 581–589. [Google Scholar] [CrossRef]
- Khan, M.; Al-Marri, A.H.; Khan, M.; Mohri, N.; Adil, S.F.; Al-Warthan, A.; Siddiqui, M.R.H.; Alkhathlan, H.Z.; Berger, R.; Tremelb, W.; et al. Pulicaria glutinosa plant extract: A green and eco-friendly reducing agent for the preparation of highly reduced graphene oxide. RSC Adv. 2014, 4, 24119–24125. [Google Scholar] [CrossRef]
- Ugarte, L.; Fernández-D’Arlas, B.; Valea, A.; González, M.L.; Corcuera, M.A.; Eceiza, A. Morphology-properties relationship in high-renewable content polyurethanes. Polym. Eng. Sci. 2014, 54, 2282–2291. [Google Scholar] [CrossRef]
- Santamaria-Echart, A.; Fernandes, I.; Saralegi, A.; Costa, M.R.P.; Barreiro, F.; Corcuera, M.A.; Eceiza, A. Synthesis of waterborne polyurethane-urea dispersions with chain extension step in homogeneous and heterogeneous media. J. Coll. Interface Sci. 2016, 476, 184–192. [Google Scholar] [CrossRef]
- Pei, A.; Malho, J.-M.; Ruokolainen, J.; Zhou, Q.; Berglund, L.A. Strong Nanocomposite Reinforcement Effects in Polyurethane Elastomer with Low Volume Fraction of Cellulose Nanocrystals. Macromolecules 2011, 44, 4422–4427. [Google Scholar] [CrossRef]
- Fuensanta, M.; Jofre-Reche, J.A.; Rodríguez-Llansola, F.; Costa, V.; Iglesias, J.I.; Martín-Martínez, J.M. Structural characterization of polyurethane ureas and waterborne polyurethane urea dispersions made with mixtures of polyester polyol and polycarbonate diol. Prog. Org. Coat. 2017, 112, 141–152. [Google Scholar] [CrossRef]
- Ou, C.-W.; Su, C.-H.; Jeng, U.-S.; Hsu, S.-H. Characterization of Biodegradable Polyurethane Nanoparticles and Thermally Induced Self-Assembly in Water Dispersion. ACS Appl. Mater. Interfaces 2014, 6, 5685–5694. [Google Scholar] [CrossRef] [PubMed]
- Strankowski, M. Effect of Variation of Hard Segment Content and Graphene-Based Nanofiller Concentration on Morphological, Thermal, and Mechanical Properties of Polyurethane Nanocomposites. Int. J. Polym. Sci. 2018, 2018, 1–20. [Google Scholar] [CrossRef]
- Nguyen, D.A.; Lee, Y.R.; Raghu, A.V.; Jeong, H.M.; Shin, C.M.; Kim, B.K. Morphological and physical properties of a thermoplastic polyurethane reinforced with functionalized graphene sheet. Polym. Int. 2009, 58, 412–417. [Google Scholar] [CrossRef]
- Auad, M.L.; Contos, V.S.; Nutt, S.; Aranguren, M.I.; Marcovich, N.E. Characterization of nanocellulose-reinforced shape memory polyurethanes. Polym. Int. 2008, 57, 651–659. [Google Scholar] [CrossRef]
- Pokharel, P.; Pant, B.; Pokhrel, K.; Pant, H.R.; Lim, J.-G.; Lee, D.S.; Kim, H.-Y.; Choi, S. Effects of functional groups on the graphene sheet for improving the thermomechanical properties of polyurethane nanocomposites. Compos. Part B Eng. 2015, 78, 192–201. [Google Scholar] [CrossRef]
- Wan, T.; Chen, D. Mechanical enhancement of self-healing waterborne polyurethane by graphene oxide. Prog. Org. Coat. 2018, 121, 73–79. [Google Scholar] [CrossRef]
- Lei, L.; Xia, Z.; Zhang, L.; Zhang, Y.; Zhong, L. Preparation and properties of amino-functional reduced graphene oxide/waterborne polyurethane hybrid emulsions. Prog. Org. Coat. 2016, 97, 19–27. [Google Scholar] [CrossRef]
- Pokharel, P.; Lee, D.S. Thermal and Mechanical Properties of Reduced Graphene Oxide/Polyurethane Nanocomposite. J. Nanosci. Nanotechnol. 2014, 14, 5718–5721. [Google Scholar] [CrossRef]
- Cho, S.Y.; Park, H.H.; Yun, Y.S.; Jin, H.-J. Influence of cellulose nanofibers on the morphology and physical properties of poly(lactic acid) foaming by supercritical carbon dioxide. Macromol. Res. 2013, 21, 529–533. [Google Scholar] [CrossRef]











| Extruder Temperature (°C) | 200 |
|---|---|
| Bed temperature (°C) | 45 |
| Nozzle diameter (mm) | 0.6 |
| Printing speed (mm/s) | 10 |
| Layer height (mm) | 0.2 |
| Infill Pattern | Rectilinear |
| Sample | Modulus (MPa) | Stress at Yield (MPa) | Stress at Break (MPa) | Strain at Break (%) |
|---|---|---|---|---|
| FWBPUU | 6.9 ± 1.3 a | 1.5 ± 0.1 a | 5.4 ± 0.6 a | 821.6 ± 35.4 a |
| F3CNF0EX | 33.3 ± 5.8 b | 5.3 ± 0.3 b | 9.1 ± 0.4 b | 341.7 ± 53.4 b,c |
| F3CNF0IN | 13.7 ± 2.9 c | 3.1 ± 0.6 c | 4.6 ± 0.8 a,c | 427.9 ± 131.0 b,d |
| F3CNF1EX | 23.8 ± 7.4 d | 3.7 ± 0.3 d | 4.6 ± 0.7 c | 394.5 ± 62.9 b,d |
| F3CNF1IN | 8.6 ± 2.3 a | 1.3 ± 0.6 a | 2.1 ± 1.2 d | 248.5 ± 136.0 c,d |
| F3GEX | 32.4 ± 5.6 b,d,e | 9.8 ± 0.9 e | 28.2 ± 1.8 e | 914.8 ± 23.8 e |
| F3GIN | 36.4 ± 6.3 b,e | 6.4 ± 0.9 f | 25.2 ± 0.9 f | 995.6 ± 59.7 f |
| Sample | Modulo (MPa) | Stress at Yield (MPa) | Stress at Break (MPa) | Strain at Break (%) |
|---|---|---|---|---|
| 3D3GEX | 30.7 ± 7.8 | 3.7 ± 0.2 | 11.4 ± 0.4 | 467.5 ± 7.3 |
| 3D3GIN | 46.7 ± 8.2 | 3.7 ± 0.3 | 12.4 ± 1.3 | 451.3 ± 29.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Larraza, I.; Vadillo, J.; Calvo-Correas, T.; Tejado, A.; Olza, S.; Peña-Rodríguez, C.; Arbelaiz, A.; Eceiza, A. Cellulose and Graphene Based Polyurethane Nanocomposites for FDM 3D Printing: Filament Properties and Printability. Polymers 2021, 13, 839. https://doi.org/10.3390/polym13050839
Larraza I, Vadillo J, Calvo-Correas T, Tejado A, Olza S, Peña-Rodríguez C, Arbelaiz A, Eceiza A. Cellulose and Graphene Based Polyurethane Nanocomposites for FDM 3D Printing: Filament Properties and Printability. Polymers. 2021; 13(5):839. https://doi.org/10.3390/polym13050839
Chicago/Turabian StyleLarraza, Izaskun, Julen Vadillo, Tamara Calvo-Correas, Alvaro Tejado, Sheila Olza, Cristina Peña-Rodríguez, Aitor Arbelaiz, and Arantxa Eceiza. 2021. "Cellulose and Graphene Based Polyurethane Nanocomposites for FDM 3D Printing: Filament Properties and Printability" Polymers 13, no. 5: 839. https://doi.org/10.3390/polym13050839
APA StyleLarraza, I., Vadillo, J., Calvo-Correas, T., Tejado, A., Olza, S., Peña-Rodríguez, C., Arbelaiz, A., & Eceiza, A. (2021). Cellulose and Graphene Based Polyurethane Nanocomposites for FDM 3D Printing: Filament Properties and Printability. Polymers, 13(5), 839. https://doi.org/10.3390/polym13050839