Complications of Poly-l-Lactic Acid and Polyglycolic Acid (PLLA/PGA) Osteosynthesis Systems for Maxillofacial Surgery: A Retrospective Clinical Investigation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.1.1. Inclusion Criteria
2.1.2. Exclusion Criteria
2.2. Data Collection
2.3. Study Outcomes
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Scheme of the Plate Placement Site and the Number of Plates at Each Site
3.3. Comparison of Background Factors between Non-Complication and Complication Group
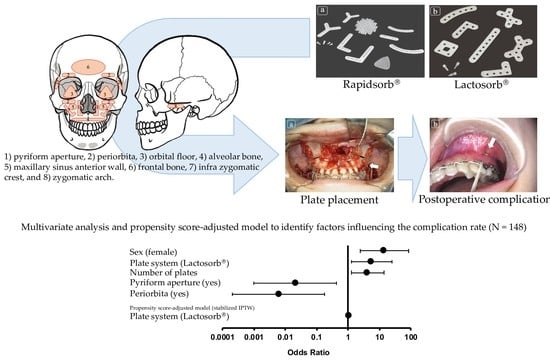
3.4. Univariate Analysis, Multivariate Analysis, and Propensity Score-Adjusted Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Kanno, T.; Sukegawa, S.; Furuki, Y.; Nariai, Y.; Sekine, J. Overview of innovative advances in bioresorbable plate systems for oral and maxillofacial surgery. Jpn. Dent. Sci. Rev. 2018, 54, 127–138. [Google Scholar] [CrossRef]
- Pihlajamaki, H.; Bostman, O.; Hirvensalo, E.; Tormala, P.; Rokkanen, P. Absorbable pins of self-reinforced poly-L-lactic acid for fixation of fractures and osteotomies. J. Bone Jt. Surg. Br. 1992, 74, 853–857. [Google Scholar] [CrossRef]
- Yolcu, U.; Alan, H.; Malkoc, S.; Bozkurt, S.B.; Hakki, S.S. Cytotoxicity evaluation of bioresorbable fixation screws on human gingival fibroblasts and mouse osteoblasts by real-Time Cell Analysis. J. Oral Maxillofac. Surg. 2015, 73, 1562.e1–1562.e10. [Google Scholar] [CrossRef]
- Schumann, P.; Lindhorst, D.; Wagner, M.E.; Schramm, A.; Gellrich, N.C.; Rucker, M. Perspectives on resorbable osteosynthesis materials in craniomaxillofacial surgery. Pathobiology 2013, 80, 211–217. [Google Scholar] [CrossRef]
- Yang, L.; Xu, M.; Jin, X.; Xu, J.; Lu, J.; Zhang, C.; Tian, T.; Teng, L. Complications of absorbable fixation in maxillofacial surgery: A meta-analysis. PLoS ONE 2013, 8, e67449. [Google Scholar] [CrossRef] [Green Version]
- Bergsma, J.E.; de Bruijn, W.C.; Rozema, F.R.; Bos, R.R.; Boering, G. Late degradation tissue response to poly(L-lactide) bone plates and screws. Biomaterials 1995, 16, 25–31. [Google Scholar] [CrossRef] [Green Version]
- Bostman, O.M.; Pihlajamaki, H.K. Late foreign-body reaction to an intraosseous bioabsorbable polylactic acid screw. A case report. J. Bone Jt. Surg. Am. 1998, 80, 1791–1794. [Google Scholar] [CrossRef] [PubMed]
- Eppley, B.L.; Reilly, M. Degradation characteristics of PLLA-PGA bone fixation devices. J. Craniofac. Surg. 1997, 8, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Pietrzak, W.S.; Eppley, B.L. Stability of craniofacial PLLA/PGA copolymer bioabsorbable screws. J. Craniofac. Surg. 2006, 17, 331–336. [Google Scholar] [CrossRef]
- Goldstein, J.A.; Quereshy, F.A.; Cohen, A.R. Early experience with biodegradable fixation for congenital pediatric craniofacial surgery. J. Craniofac. Surg. 1997, 8, 110–115. [Google Scholar] [CrossRef]
- Sukegawa, S.; Kanno, T.; Matsumoto, K.; Sukegawa-Takahashi, Y.; Masui, M.; Furuki, Y. Complications of a poly-L-lactic acid and polyglycolic acid osteosynthesis device for internal fixation in maxillofacial surgery. Odontology 2018, 106, 360–368. [Google Scholar] [CrossRef]
- Toro, C.; Robiony, M.; Zerman, N.; Politi, M. Resorbable plates in maxillary fixation. A 5-year experience. Minerva Stomatol. 2005, 54, 199–206. [Google Scholar] [PubMed]
- Norholt, S.E.; Pedersen, T.K.; Jensen, J. Le Fort I miniplate osteosynthesis: A randomized, prospective study comparing resorbable PLLA/PGA with titanium. Int. J. Oral Maxillofac. Surg. 2004, 33, 245–252. [Google Scholar] [CrossRef]
- Zhu, J.; Sharma, D.B.; Gray, S.W.; Chen, A.B.; Weeks, J.C.; Schrag, D. Carboplatin and paclitaxel with vs without bevacizumab in older patients with advanced non-small cell lung cancer. JAMA 2012, 307, 1593–1601. [Google Scholar] [CrossRef] [PubMed]
- Lunceford, J.K.; Davidian, M. Stratification and weighting via the propensity score in estimation of causal treatment effects: A comparative study. Stat. Med. 2004, 23, 2937–2960. [Google Scholar] [CrossRef]
- Robins, J.M.; Hernan, M.A.; Brumback, B. Marginal structural models and causal inference in epidemiology. Epidemiology 2000, 11, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Kelley, P.; Crawford, M.; Higuera, S.; Hollier, L.H. Two hundred ninety-four consecutive facial fractures in an urban trauma center: Lessons learned. Plast. Reconstr. Surg. 2005, 116, 42e–49e. [Google Scholar] [CrossRef]
- Kirkpatrick, D.; Gandhi, R.; Van Sickels, J.E. Infections associated with locking reconstruction plates: A retrospective review. J. Oral Maxillofac. Surg. 2003, 61, 462–466. [Google Scholar] [CrossRef]
- Bell, R.B.; Kindsfater, C.S. The use of biodegradable plates and screws to stabilize facial fractures. J. Oral Maxillofac. Surg. 2006, 64, 31–39. [Google Scholar] [CrossRef]
- Murthy, A.S.; Lehman, J.A., Jr. Symptomatic plate removal in maxillofacial trauma: A review of 76 cases. Ann. Plast. Surg. 2005, 55, 603–607. [Google Scholar] [CrossRef]
- Furr, A.M.; Schweinfurth, J.M.; May, W.L. Factors associated with long-term complications after repair of mandibular fractures. Laryngoscope 2006, 116, 427–430. [Google Scholar] [CrossRef]
- Torgerson, D.J.; Bell-Syer, S.E. Hormone replacement therapy and prevention of nonvertebral fractures: A meta-analysis of randomized trials. JAMA 2001, 285, 2891–2897. [Google Scholar] [CrossRef] [PubMed]
- Szabo, A.; Janovszky, A.; Pocs, L.; Boros, M. The periosteal microcirculation in health and disease: An update on clinical significance. Microvasc. Res. 2017, 110, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Doh, G.; Bahk, S.; Hong, K.Y.; Lim, S.; Han, K.M.; Eo, S. Delayed formation of sterile abscess after zygomaticomaxillary complex fracture treatment with bioabsorbable plates. Arch. Craniofac. Surg. 2018, 19, 143–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wijdicks, F.J.; Van der Meijden, O.A.; Millett, P.J.; Verleisdonk, E.J.; Houwert, R.M. Systematic review of the complications of plate fixation of clavicle fractures. Arch. Orthop. Trauma Surg. 2012, 132, 617–625. [Google Scholar] [CrossRef] [Green Version]
- Francel, T.J.; Birely, B.C.; Ringelman, P.R.; Manson, P.N. The fate of plates and screws after facial fracture reconstruction. Plast. Reconstr. Surg. 1992, 90, 568–573. [Google Scholar] [CrossRef]
- Stone, I.E.; Dodson, T.B.; Bays, R.A. Risk factors for infection following operative treatment of mandibular fractures: A multivariate analysis. Plast. Reconstr. Surg. 1993, 91, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Nagase, D.Y.; Courtemanche, D.J.; Peters, D.A. Plate removal in traumatic facial fractures: 13-year practice review. Ann. Plast. Surg. 2005, 55, 608–611. [Google Scholar] [CrossRef]
- O'Connell, J.; Murphy, C.; Ikeagwuani, O.; Adley, C.; Kearns, G. The fate of titanium miniplates and screws used in maxillofacial surgery: A 10 year retrospective study. Int. J. Oral Maxillofac. Surg. 2009, 38, 731–735. [Google Scholar] [CrossRef]
- Wan, K.; Williamson, R.A.; Gebauer, D.; Hird, K. Open reduction and internal fixation of mandibular angle fractures: Does the transbuccal technique produce fewer complications after treatment than the transoral technique? J. Oral Maxillofac. Surg. 2012, 70, 2620–2628. [Google Scholar] [CrossRef]
- Ding, L.; He, Z.; Xiao, H.; Chai, L.; Xue, F. Risk factors for postoperative wound complications of calcaneal fractures following plate fixation. Foot Ankle Int. 2013, 34, 1238–1244. [Google Scholar] [CrossRef]
- Sukegawa, S.; Kanno, T.; Nagano, D.; Shibata, A.; Sukegawa-Takahashi, Y.; Furuki, Y. The clinical feasibility of newly developed thin flat-type bioresorbable osteosynthesis devices for the internal fixation of zygomatic fractures: Is there a difference in healing between bioresorbable materials and titanium osteosynthesis? J. Craniofac. Surg. 2016, 27, 2124–2129. [Google Scholar] [CrossRef] [Green Version]
- Islamoglu, K.; Coskunfirat, O.K.; Tetik, G.; Ozgentas, H.E. Complications and removal rates of miniplates and screws used for maxillofacial fractures. Ann. Plast. Surg. 2002, 48, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Rallis, G.; Mourouzis, C.; Papakosta, V.; Papanastasiou, G.; Zachariades, N. Reasons for miniplate removal following maxillofacial trauma: A 4-year study. J. Craniomaxillofac. Surg. 2006, 34, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, V.; Chhabra, P.; Dover, M.S. Removal of miniplates in maxillofacial surgery: A follow-up study. J. Oral Maxillofac. Surg. 2005, 63, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Hernandez Rosa, J.; Villanueva, N.L.; Sanati-Mehrizy, P.; Factor, S.H.; Taub, P.J. Review of maxillofacial hardware complications and indications for salvage. Craniomaxillofac. Trauma Reconstr. 2016, 9, 134–140. [Google Scholar] [CrossRef] [Green Version]



| Characteristics | n (%) or Median (IQR) | |||
|---|---|---|---|---|
| Total (n = 148) | RapidSorb® (n = 61) | Lactosorb® (n = 87) | ||
| Age | 37.5 (21.3–72.0) | 42.0 (24.5–74.0) | 34.0 (18.0–68.0) | |
| Sex | Male | 81 (54.7) | 35 (57.4) | 46 (52.9) |
| Female | 67 (45.3) | 26 (42.6) | 41 (47.1) | |
| Brinkman index | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | |
| Type 2 diabetes mellitus (yes) | 8 (5.4) | 5 (8.2) | 3 (3.4) | |
| Osteoporosis (yes) | 6 (4.1) | 2 (3.3) | 4 (4.6) | |
| Steroid (yes) | 3 (2.0) | 1 (1.6) | 2 (2.3) | |
| Diagnosis | Midfacial fracture/trauma | 101 (68.2) | 42 (68.9) | 59 (67.8) |
| Dentofacial deformity | 47 (31.8) | 19 (31.1) | 28 (32.2) | |
| Type of surgery for internal fixation | Le Fort I osteotomy | 41 (27.7) | 16 (26.2) | 25 (28.7) |
| Maxillary anterior osteotomy | 6 (4.1) | 3 (4.9) | 3 (3.4) | |
| Maxillary fracture | 24 (16.2) | 7 (11.5) | 17 (19.5) | |
| Zygomatic arch/zygomatic fracture | 38 (25.7) | 20 (32.8) | 18 (20.7) | |
| Multiple midfacial fracture | 30 (20.3) | 13 (21.3) | 17 (19.5) | |
| Orbital floor fracture | 4 (2.7) | 2 (3.3) | 2 (2.3) | |
| Naso-orbito-ethmoidal fracture | 3 (2.0) | 0 (0.0) | 3 (3.4) | |
| Frontal bone fracture | 2 (1.4) | 0 (0.0) | 2 (2.3) | |
| Number of plates | 2.5 (2.0–4.0) | 3.0 (2.0–4.0) | 2.0 (1.0–4.0) | |
| Thickness of plates | 1.5 mm | 45 (30.4) | 11 (18.0) | 34 (39.1) |
| 2.0 mm | 103 (69.6) | 50 (82.0) | 53 (60.9) | |
| Site of the plate placement (yes) | Pyriform aperture | 75 (50.7) | 30 (49.2) | 45 (51.7) |
| Periorbita | 58 (39.2) | 30 (49.2) | 28 (32.2) | |
| Orbital floor | 3 (2.0) | 0 (0.0) | 3 (3.4) | |
| Alveolar bone | 11 (7.4) | 5 (8.2) | 6 (6.9) | |
| Maxillary sinus anterior wall | 12 (8.1) | 5 (8.2) | 7 (8.0) | |
| Frontal bone | 2 (1.4) | 0 (0.0) | 2 (2.3) | |
| Infra zygomatic crest | 96 (64.9) | 44 (72.1) | 52 (59.8) | |
| Zygomatic arch | 13 (8.8) | 6 (9.8) | 7 (8.0) | |
| Characteristics | n (%) or Median (Interquartile Range: IQR) | |||
|---|---|---|---|---|
| Non-Complication (n = 132) | Complication (n = 16) | p-Value | ||
| Age | 40.0 (22.0–74.0) | 27.5 (17.3–46.0) | 0.08 a | |
| Sex | Male | 79 (59.8) | 2 (12.5) | <0.01 *b |
| Female | 53 (40.2) | 14 (87.5) | ||
| Brinkman index | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.60 a | |
| Type 2 diabetes mellitus (yes) | 8 (6.1) | 0 (0.0) | 0.60 b | |
| Osteoporosis (yes) | 6 (4.5) | 0 (0.0) | 1.00 b | |
| Steroid (yes) | 3 (2.3) | 0 (0.0) | 1.00 b | |
| Diagnosis | Midfacial fracture/trauma | 95 (72.0) | 6 (37.5) | <0.01 *b |
| Dentofacial deformity | 37 (28.0) | 10 (62.5) | ||
| Type of surgery for internal fixation | Le Fort I osteotomy | 31 (23.5) | 10 (62.5) | - |
| Maxillary anterior osteotomy | 6 (4.5) | 4 (25.0) | ||
| Maxillary fracture | 20 (15.2) | 0 (0.0) | ||
| Zygomatic arch/zygomatic fracture | 38 (28.8) | 0 (0.0) | ||
| Multiple midfacial fracture | 28 (21.2) | 2 (12.5) | ||
| Orbital floor fracture | 4 (3.0) | 0 (0.0) | ||
| Naso-orbito-ethmoidal fracture | 3 (2.3) | 0 (0.0) | ||
| Frontal bone fracture | 2 (1.5) | 0 (0.0) | ||
| Plate system | RapidSorb® | 58 (43.9) | 3 (18.8) | 0.06 b |
| Lactosorb® | 74 (56.1) | 13 (81.3) | ||
| Number of plates | 2.0 (2.0–3.8) | 4.0 (2.0–4.0) | 0.05 a | |
| Thickness of plates | 1.5 mm | 43 (32.6) | 2 (12.5) | 0.15 b |
| 2.0 mm | 89 (67.4) | 14 (87.5) | ||
| Site of the plate placement | Pyriform aperture | 65 (49.2) | 10 (62.5) | 0.43 b |
| Periorbita | 57 (43.2) | 1 (6.3) | <0.01 *b | |
| Orbital floor | 3 (2.3) | 0 (0.0) | 1.00 b | |
| Alveolar bone | 9 (6.8) | 2 (12.5) | 0.34 b | |
| Maxillary sinus anterior wall | 11 (8.3) | 1 (6.3) | 1.00 b | |
| Frontal bone | 2 (1.5) | 0 (0.0) | 1.00 b | |
| Infra zygomatic crest | 83 (62.9) | 13 (81.3) | 0.18 b | |
| Zygomatic arch | 12 (9.1) | 1 (6.3) | 1.00 b | |
| Type of complication | Plate exposure | - | 11 (68.8) | - |
| Infection | - | 4 (25.0) | - | |
| Plate breakage | - | 1 (6.3) | - | |
| Complication site | Pyriform aperture | - | 2 (12.5) | - |
| Alveolar bone | - | 2 (12.5) | - | |
| Maxillary sinus anterior wall | - | 1 (6.3) | - | |
| Infra zygomatic crest | - | 11 (68.8) | - | |
| Complication date (day) | - | 81.5 (43.8–128.8) | - | |
| Characteristics | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Age | 0.98 (0.95–1.00) | 0.06 | ||
| Sex (female) | 10.43 (2.28–47.80) | <0.01 * | 14.27 (2.42–84.27) | <0.01 * |
| Diagnosis (dentofacial deformity) | 4.28 (1.45–12.61) | <0.01 * | ||
| Type 2 diabetes mellitus (yes) | 0.00 (0.00) | 1.00 | ||
| Osteoporosis (yes) | 0.00 (0.00) | 1.00 | ||
| Steroid (yes) | 0.00 (0.00) | 1.00 | ||
| Brinkman index | 1.00 (0.99–1.00) | 0.45 | ||
| Plate system (Lactosorb®) | 3.40 (0.92–12.48) | 0.07 | 5.58 (1.27–24.45) | 0.02 * |
| Thickness of plate (2.0 mm) | 3.38 (0.74–15.55) | 0.12 | ||
| Number of plates | 1.47 (0.96–2.25) | 0.08 | 4.17 (1.26–13.82) | 0.02 * |
| Pyriform aperture (yes) | 1.72 (0.59–5.00) | 0.32 | 0.02 (0.001–0.43) | 0.01 * |
| Periorbita (yes) | 0.09 (0.01–0.68) | 0.02 * | 0.006 (0.0002–0.173) | <0.01 * |
| Orbital floor (yes) | 0.00 (0.00) | 1.00 | ||
| Alveolar bone (yes) | 1.95 (0.38–9.95) | 0.42 | ||
| Maxillary sinus anterior wall (yes) | 0.73 (0.09–6.09) | 0.77 | ||
| Frontal bone (yes) | 0.00 (0.00) | 1.00 | ||
| Infra zygomatic crest (yes) | 2.56 (0.69–9.43) | 0.16 | ||
| Zygomatic arch (yes) | 0.67 (0.08–5.50) | 0.71 | ||
| Propensity score-adjusted model (stabilized IPTW) | ||||
| Plate system (Lactosorb®) | 1.007 (1.001–1.055) | <0.01 * | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsuda, Y.; Karino, M.; Okui, T.; Kanno, T. Complications of Poly-l-Lactic Acid and Polyglycolic Acid (PLLA/PGA) Osteosynthesis Systems for Maxillofacial Surgery: A Retrospective Clinical Investigation. Polymers 2021, 13, 889. https://doi.org/10.3390/polym13060889
Matsuda Y, Karino M, Okui T, Kanno T. Complications of Poly-l-Lactic Acid and Polyglycolic Acid (PLLA/PGA) Osteosynthesis Systems for Maxillofacial Surgery: A Retrospective Clinical Investigation. Polymers. 2021; 13(6):889. https://doi.org/10.3390/polym13060889
Chicago/Turabian StyleMatsuda, Yuhei, Masaaki Karino, Tatsuo Okui, and Takahiro Kanno. 2021. "Complications of Poly-l-Lactic Acid and Polyglycolic Acid (PLLA/PGA) Osteosynthesis Systems for Maxillofacial Surgery: A Retrospective Clinical Investigation" Polymers 13, no. 6: 889. https://doi.org/10.3390/polym13060889
APA StyleMatsuda, Y., Karino, M., Okui, T., & Kanno, T. (2021). Complications of Poly-l-Lactic Acid and Polyglycolic Acid (PLLA/PGA) Osteosynthesis Systems for Maxillofacial Surgery: A Retrospective Clinical Investigation. Polymers, 13(6), 889. https://doi.org/10.3390/polym13060889