Immobilization of Caraway Essential Oil in a Polypropylene Matrix for Antimicrobial Modification of a Polymeric Surface
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
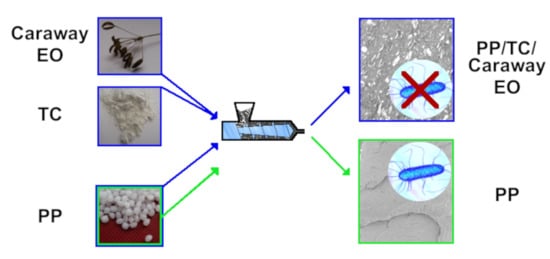
2.2. Preparation of Samples
2.3. Morphological Studies
2.3.1. Scanning Electron Microscopy
2.3.2. Tensile Properties
2.4. Thermal Properties
2.4.1. Thermogravimetric Analysis
2.4.2. Differential Scanning Calorimetry
2.5. Qualitative Analysis of the Caraway EO
2.6. Release Kinetics of the Caraway EO
2.7. Study of Antimicrobial Properties
2.7.1. Disk Diffusion Method
2.7.2. Measurement of Antibacterial Activity on the Plastic Surfaces
3. Results and Discussion
3.1. Morphological Studies
3.1.1. Scanning Electron Microscopy
3.1.2. Tensile Properties
3.2. Thermal Properties
3.2.1. Thermogravimetric Analysis
3.2.2. Differential Scanning Calorimetry
3.3. Qualitative Analysis of the Caraway EO
3.4. Release Kinetics of the Caraway EO
3.5. Study of Antimicrobial Properties
3.5.1. Disk Diffusion Method
3.5.2. Measurement of Antibacterial Activity on the Plastic Surfaces
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eurostat. Available online: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/DDN-20170412-1 (accessed on 4 December 2020).
- Bintsis, T. Microbial pollution and food safety. Aims Microbiol. 2018, 4, 377–396. [Google Scholar] [CrossRef]
- Machado-Moreira, B.; Richards, K.; Brennan, F.; Abram, F.; Burgess, C.M. Microbial Contamination of Fresh Produce: What, Where, and How? Compr. Rev. Food Sci. Food Saf. 2019, 18, 1727–1750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jampilek, J.; Kraľova, K. Chapter 17—Nanobiopesticides in agriculture: State of the art and future. In Nano-Biopesticides Today and Future Perspectives, 1st ed.; Koul, O., Ed.; Academic Press: London, UK, 2019; pp. 397–447. [Google Scholar]
- Ramos, M.; Jimenez, A.; Garrigos, M.C. Active nanocomposites in food contact materials. In Nanoscience in Food and Agriculture 4, 1st ed.; Ranjan, S., Dasgupta, N., Lichtfouse, E., Eds.; Springer International Publishing AG: Cham, Switzerland, 2017; Volume 4, pp. 1–44. [Google Scholar]
- Prakash, B.; Kumar, A.; Pratap, P.; Songachan, S.L.S. 1—Antimicrobial and antioxidant properties of phytochemicals: Current status and future perspective. In Functional and Preservative Properties of Phytochemicals, 1st ed.; Prakash, B., Ed.; Academic Press: London, UK, 2020; pp. 1–45. [Google Scholar]
- Scaffaro, R.; Maio, A.; Gulino, E.F.; Morreale, M.; La Mantia, F.P. The Effects of Nanoclay on the Mechanical Properties, Carvacrol Release and Degradation of a PLA/PBAT Blend. Materials 2020, 13, 983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emamifar, A.; Kadivar, M.; Shahedi, M.; Soleimanian-Zad, S. Evaluation of nanocomposite packaging containing Ag and ZnO on shelf life of fresh orange juice. Innov. Food Sci. Emerg. Technol. 2010, 11, 742–748. [Google Scholar] [CrossRef]
- Bhavaniramya, S.; Vishnupriya, S.; Al-Aboody, M.S.; Vijayakumar, R.; Baskaran, D. Role of essential oils in food safety: Antimicrobial and antioxidant applications. Grain Oil Sci. Technol. 2019, 2, 49–55. [Google Scholar] [CrossRef]
- Moro, I.J.; Gondo, G.D.G.A.; Pierri, E.G.; Rodrigues Pietro, C.L.; Pienna Soares, C.; Pergentino de Sousa, D.; Gonzaga dos Santos, A. Evaluation of antimicrobial, cytotoxic and chemopreventive activities of carvone and its derivatives. Braz. J. Pharm. Sci. 2017, 53, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Karimirad, R.; Behnamian, M.; Dezhsetan, S. Application of chitosan nanoparticles containing Cuminum cyminum oil as a delivery system for shelf life extension of Agaricus bisporus. LWT 2019, 106, 218–228. [Google Scholar] [CrossRef]
- Boughendjioua, H. Characterization of aroma active compounds of cumin (Cuminum cyminum, L.) seed essential oil. Mod. Appl. Bioequivalence Bioavailab. 2019, 4, 1–5. [Google Scholar]
- Bouwmeester, H.J.; Gershenzon, J.; Konings, M.C.; Croteau, R. Biosynthesis of the Monoterpenes Limonene and Carvone in the Fruit of Caraway. Plant. Physiol. 1998, 117, 901–912. [Google Scholar] [CrossRef] [Green Version]
- Laribi, B.; Bettaieb, I.; Kouki, K.; Sahli, A.; Mougou, A.; Marzouk, B. Water deficit effects on caraway (Carum carvi L.) growth, essential oil and fatty acid composition. Ind. Crop. Prod. 2009, 30, 372–379. [Google Scholar] [CrossRef]
- Muthuraj, R.; Misra, M.; Mohanty, A.K. Biodegradable compatibilized polymer blends for packaging applications: A lit-erature review. J. Appl. Polym. Sci. 2017, 135, 1–35. [Google Scholar]
- Hahladakis, J.N.; Iacovidou, E.; Gerassimidou, S. Chapter 19—Plastic waste in a circular economy. In Plastics Waste and Recycling: Environmental Impact, Societal Issues, Prevention, and Solutions, 1st ed.; Letcher, T.M., Ed.; Academic Press: London, UK, 2020; pp. 481–512. [Google Scholar]
- Abdelwahab, M.A.; Rodriguez-Uribe, A.; Misra, M.; Mohanty, A.K. Injection molded novel biocomposites from polypro-pylene and sustainable biocarbon. Molecules 2019, 24, 4026. [Google Scholar] [CrossRef] [Green Version]
- Ulusoy, U. Application of ANOVA to image analysis results of talc particles produced by different milling. Powder Technol. 2008, 188, 133–138. [Google Scholar] [CrossRef]
- Jadhav, N.R.; Paradkar, A.; Salunkhe, N.H.; Karade, R.S.; Mane, G.G. Talc: A versatile pharmaceutical excipient. WJPPS 2013, 2, 4639–4660. [Google Scholar]
- Keawchaoon, L.; Yoksan, R. Preparation, characterization and in vitro release study of carvacrol-loaded chitosan nano-particles. Colloids Surf. B Biointerfaces 2011, 84, 163–171. [Google Scholar] [CrossRef]
- Licciardello, F.; Muratore, G.; Mercea, P.; Tosa, V.; Nerin, C. Diffusional Behaviour of Essential Oil Components in Active Packaging Polypropylene Films by Multiple Headspace Solid Phase Microextraction-Gas Chromatography. Packag. Technol. Sci. 2012, 26, 173–185. [Google Scholar] [CrossRef]
- Honarvar, Z.; Farhoodi, M.; Khani, M.R.; Mohammadi, A.; Shokri, B.; Ferdowsi, R.; Shojaee-Aliabadi, S. Application of cold plasma to develop carboxymethyl cellulose-coated polypropylene films containing essential oil. Carbohydr. Polym. 2017, 176, 1–10. [Google Scholar] [CrossRef]
- Llana-Ruiz-Cabello, M.; Pichardo, S.; Bermudez, J.M.; Baños, A.; Ariza, J.J.; Guillamon, E.; Aucejo, S.; Camean, A.M. Characterization and antimicrobial activity of active polypropylene films containing Oregano essential oil and Allium ex-tract to be used in packaging for meat products. Food Addit. Contam. Part A 2018, 35, 782–791. [Google Scholar] [CrossRef] [PubMed]
- Alonso, Y.N.; Grafia, A.L.; Castillo, L.A.; Barbosa, S.E. Lemon essential oil desorption from polypropylene/talc nanocom-posite films. Iran. Polym. J. 2016, 25, 999–1008. [Google Scholar] [CrossRef]
- ASTM D882-18. Standard Test Method for Tensile Properties of Thin Plastic Sheeting; ASTM International: West Con-shohocken, PA, USA, 2012. [Google Scholar] [CrossRef]
- Ammar, O.; Bouaziz, Y.; Haddar, N.; Mnif, N. Talc as reinforcing filler in polypropylene compounds: Effect on morphology and mechanical properties. Polym. Sci. 2017, 3, 1–7. [Google Scholar]
- Patnaik, K.S.K.R.; Devi, K.S.; Kumar, V.K. Non-isothermal Crystallization Kinetics of Polypropylene (PP) and Polypropylene (PP)/Talc Nanocomposite. Int. J. Chem. Eng. Appl. 2010, 1, 346–353. [Google Scholar] [CrossRef]
- Akgün, M.; Başaran, I.; Suner, S.C.; Oral, A. Geraniol and cinnamaldehyde as natural antibacterial additives for poly(lactic acid) and their plasticizing effects. J. Polym. Eng. 2019, 40, 38–48. [Google Scholar] [CrossRef]
- EUCAST: Disk Diffusion Methodology. Available online: https://www.eucast.org/ast_of_bacteria/disk_diffusion_methodology/ (accessed on 27 May 2020).
- ISO 22196. Plastics-Measurement of Antibacterial Activity on Plastics Surfaces; International Organization of Standardiza-tion: Geneva, Switzerland, 1 August 2011. [Google Scholar]
- Deshmukh, K.A.; Pode, G.R.; Roy, S.R.; Gupte, B.K.; Deshmukh, A.D.; Chopra, S.; Peshwe, D.R. Effect of Cryo-Ageing at Liquid Nitrogen Temperature and Subsequent Thermal-Annealing on the Interface of Talc Filled Polypropylene with Different Particle Size. Trans. Indian Inst. Met. 2017, 71, 403–409. [Google Scholar] [CrossRef]
- Castillo, A.L.; Barbosa, S.E. Influence of processing and particle morphology on final properties of polypropylene/talc nanocomposites. Polym. Compos. 2020, 41, 3170–3183. [Google Scholar] [CrossRef]
- Sasimowski, E.; Majewski, Ł.; Grochowicz, M. Influence of the Conditions of Corotating Twin-Screw Extrusion for Talc-Filled Polypropylene on Selected Properties of the Extrudate. Polymers 2019, 11, 1460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urbankova, M.; Hrabalikova, M.; Poljansek, I.; Miskolczi, N.; Sedlarik, V. Antibacterial polymer composites based on low-density polyethylene and essential oils immobilized on various solid carriers. J. Appl. Polym. Sci. 2015, 132, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Chang, T.; Dong, S.; Zhang, D.; Ma, C.; Chen, S.; Li, H. Biopolymer films based on chitosan/potato protein/linseed oil/ZnO NPs to maintain the storage quality of raw meat. Food Chem. 2020, 332, 127375. [Google Scholar] [CrossRef] [PubMed]
- Lian, H.; Shi, J.; Zhang, X.; Peng, Y. Effect of the added polysaccharide on the release of thyme essential oil and structure properties of chitosan based film. Food Packag. Shelf Life 2020, 23, 100467. [Google Scholar] [CrossRef]
- Shubhra, Q.T.H.; Akmm, A.; Ma, Q. Mechanical properties of polypropylene composites: A review. J. Thermoplast Compos. Mater. 2011, 26, 362–391. [Google Scholar] [CrossRef]
- Yiga, V.A.; Pagel, S.; Lubwama, M.; Epple, S.; Wilberforce Olupot, P.; Bonten, C. Development of fiberreinforced poly-propylene with NaOH pretreated rice and coffee husks as fillers: Mechanical and thermal properties. J. Thermoplast Compos. Mater. 2019, 33, 1269–1291. [Google Scholar] [CrossRef]
- Wang, G.; Zhao, G.; Dong, G.; Mu, Y.; Park, C.B. Lightweight and strong microcellular injection molded PP/talc nano-composite. Compos. Sci. Technol. 2018, 168, 38–46. [Google Scholar] [CrossRef]
- Karrad, S.; Lopez Cuesta, J.M.; Crespy, A. Influence of a fine talc on the properties of composites with high density poly-ethylene and polyethylene/polystyrene blends. J. Mater. Sci. 1998, 33, 453–461. [Google Scholar] [CrossRef]
- Šuput, D.; Lazić, V.; Pezo, L.; Markov, S.; Vaštag, Ž.; Popović, L.; Radulović, A.; Ostojić, S.; Zlatanović, S.; Popović, S. Characterization of Starch Edible Films with Different Essential Oils Addition. Pol. J. Food Nutr. Sci. 2016, 66, 277–285. [Google Scholar] [CrossRef]
- Souza, A.; Goto, G.; Mainardi, J.; Coelho, A.; Tadini, C. Cassava starch composite films incorporated with cinnamon essential oil: Antimicrobial activity, microstructure, mechanical and barrier properties. LWT 2013, 54, 346–352. [Google Scholar] [CrossRef]
- Mekonnen, T.; Mussone, P.G.; Khalil, H.; Bressler, D.C. Progress in bio-based plastics and plasticizing modifications. J. Mater. Chem. A 2013, 1, 13379–13398. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Qin, Y.; Yuan, M.; Li, L.; Chen, H.; Cao, J.; Yang, J. Characterization of an antimicrobial poly(lactic acid) film prepared with poly(ε-caprolactone) and thymol for active packaging. Polym. Adv. Technol. 2014, 25, 948–954. [Google Scholar] [CrossRef]
- Mandal, D.K.; Bhunia, H.; Bajpai, P.K.; Bhalla, V.K. Thermal degradation kinetics and estimation of lifetime of radiation grafted polypropylene films. Radiat. Phys. Chem. 2017, 136, 1–8. [Google Scholar] [CrossRef]
- Yavuz, H.; Rzaev, Z.; Dilsiz, N. Characterisation of flame retardant plasma polymer deposited BOPP film. Plast. Rubber Compos. 2008, 37, 268–275. [Google Scholar] [CrossRef]
- Minkova, L.; Lefterova, L.; Koleva, T.; Nedkov, E.; Nikolova, M. Thermogravimetry and differential scanning calorimetry of γ-irradiated i-polypropylene films. Colloid Polym. Sci. 1988, 266, 898–905. [Google Scholar] [CrossRef]
- Longo, C.; Savaris, M.; Zeni, M.; Brandalise, R.N.; Grisa, A.M.C. Degradation study of polypropylene (PP) and bioriented polypropylene (BOPP) in the environment. Mater. Res. 2011, 14, 442–448. [Google Scholar] [CrossRef] [Green Version]
- Tsuge, S.; Ohtani, H.; Watanabe, C. Pyrolysis-GC/MS Data Book of Synthetic Polymers—Pyrograms, Thermograms and MS of Pyrolyzates, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 12–20. [Google Scholar]
- Kusch, P. Pyrolysis-Gas Chromatography/Mass Spectrometry of Polymeric Materials; IntechOpen: London, UK, 2012; pp. 35–38. [Google Scholar]
- Schöne, J.; Kotter, I.; Grellmann, W. Properties of polypropylene talc compounds with different talc particle size and loading. J. Plast. Technol. 2012, 8, 230–251. [Google Scholar]
- Nofar, M.; Ozgen, E.; Girginer, B. Injection-molded PP composites reinforced with talc and nanoclay for automotive ap-plications. J. Thermoplast Compos. Mater. 2019, 33, 1–21. [Google Scholar]
- Marques, M.D.F.V.; Poloponsky, M.; Chaves, É.G. Influence of the elastomeric polypropylene addition on the properties of commercial metallocenic polypropylene. Mater. Res. 2001, 4, 251–254. [Google Scholar] [CrossRef] [Green Version]
- Ramos, M.; Jiménez, A.; Peltzer, M.; Garrigós, M.C. Characterization and antimicrobial activity studies of polypropylene films with carvacrol and thymol for active packaging. J. Food Eng. 2012, 109, 513–519. [Google Scholar] [CrossRef]
- Laribi, B.; Kouki, K.; Bettaieba, T.; Mougou, A.; Marzouk, B. Comparative GC analysis of seed essential oils from tunisian and german caraway (Carum carvi L.) ecotypes. Phcog. Commn. 2012, 2, 52–57. [Google Scholar]
- Iacobellis, N.S.; LO Cantore, P.; Capasso, F.; Senatore, F. Antibacterial activity of Cuminum cyminum L. and Carum carvi L. essential oils. J. Agric. Food Chem. 2005, 53, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Solberg, S.O.; Göransson, M.; Petersen, M.A.; Yndgaard, F.; Jeppson, S. Caraway essential oil composition and morphology: The role of location and genotype. Biochem. Syst. Ecol. 2016, 66, 351–357. [Google Scholar] [CrossRef]
- Chemat, S.; Aït-Amar, H.; Lagha, A.; Esveld, D. Microwave-assisted extraction kinetics of terpenes from caraway seeds. Chem. Eng. Process. Process. Intensif. 2005, 44, 1320–1326. [Google Scholar] [CrossRef]
- Hromiš, N.M.; Lazić, V.L.; Markov, S.L.; Vaštag, Ž.G.; Popović, S.Z.; Šuput, D.Z.; Džinić, N.R.; Velićanski, A.S.; Popović, L.M. Optimization of chitosan biofilm properties by addition of caraway essential oil and beeswax. J. Food Eng. 2015, 158, 86–93. [Google Scholar] [CrossRef]
- Wu, J.; Sun, X.; Guo, X.; Ge, S.; Zhang, Q. Physicochemical properties, antimicrobial activity and oil release of fish gelatin films incorporated with cinnamon essential oil. Aquac. Fish. 2017, 2, 185–192. [Google Scholar] [CrossRef]
- Tunç, S.; Duman, O. Preparation of active antimicrobial methyl cellulose/carvacrol/montmorillonite nanocomposite films and investigation of carvacrol release. LWT 2011, 44, 465–472. [Google Scholar] [CrossRef]
- Naveed, R.; Hussain, I.; Tawab, A.; Tariq, M.; Rahman, M.; Hameed, S.; Mahmood, M.S.; Siddique, A.B.; Iqbal, M. Antimicrobial activity of the bioactive components of essential oils from Pakistani spices against Salmonella and other multi-drug resistant bacteria. BMC Complement. Altern. Med. 2013, 13, 265. [Google Scholar] [CrossRef] [Green Version]
- Trombetta, D.; Castelli, F.; Sarpietro, M.G.; Venuti, V.; Cristani, M.; Daniele, C.; Saija, A.; Mazzanti, G.; Bisignano, G. Mechanisms of Antibacterial Action of Three Monoterpenes. Antimicrob. Agents Chemother. 2005, 49, 2474–2478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of Essential Oils on Pathogenic Bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, H.A. Essential Oil as Antimicrobial Agents: Efficacy, Stability, and Safety Issues for Food Application. In Essential Oils—Bioactive Compounds, New Perspectives and Applications; IntechOpen: London, UK, 2020. [Google Scholar]
- Feng, K.; Wen, P.; Yang, H.; Li, N.; Lou, W.Y.; Zong, M.H.; Wu, H. Enhancement of the antimicrobial activity of cinnamon essential oil-loaded electrospun nanofilm by the incorporation of lysozyme. RSC Adv. 2017, 7, 1572–1580. [Google Scholar] [CrossRef] [Green Version]
- Perez, M.A.; Rivas, B.L.; Garrido-Miranda, K.A.; Campos-Requena, V.H.; Martinez, M.; Castano, J.; Maldonado, A. Low density polymethylene (LDPE) nanocomposites with passive and active barrier properties. J. Chil. Chem. Soc. 2014, 59, 2442–2446. [Google Scholar]
- Kneuer, C.; Sameti, M.; Haltner, E.G.; Schiestel, T.; Schirra, H.; Schmidt, H.; Lehr, C.-M. Silica nanoparticles modified with aminosilanes as carriers for plasmid DNA. Int. J. Pharm. 2000, 196, 257–261. [Google Scholar] [CrossRef] [Green Version]






| Designation | PP (wt%) | TC (wt%) | Theoretical EO Content (wt%) | Actual EO Content (wt%) 1 |
|---|---|---|---|---|
| PP | 100 | - | - | - |
| PP/TC | 70 | 30 | - | - |
| PP/TC/3EO | 67 | 30 | 3 | 0.9 ± 0.1 |
| PP/TC/7EO | 63 | 30 | 7 | 1.7 ± 0.2 |
| PP/TC/10EO | 60 | 30 | 10 | 4.9 ± 0.2 |
| PP/TC/20EO | 40 | 30 | 20 | 7.1 ± 1.2 |
| Sample | Tm (°C) | Tc (°C) | ∆Hf (J/g) | XC (%) |
|---|---|---|---|---|
| PP | 164.4 | 127.9 | −98.3 | 47.0 |
| PP/TC | 165.4 | 128.1 | −83,8 | 57.3 |
| PP/TC/3EO | 164.9 | 128.4 | −59.3 | 41.0 |
| PP/TC/7EO | 164.9 | 128.7 | −60.4 | 42.3 |
| PP/TC/10EO | 164.5 | 127.3 | −63.6 | 46.6 |
| PP/TC/20EO | 164.8 | 128.5 | −44.9 | 34.1 |
| Peak # | Retention Time (min) | Retention Index | CAS # | Name | Mw | Area (%) |
|---|---|---|---|---|---|---|
| 1 | 7.274 | 958 | 123-35-3 | beta-Myrcene | 136 | ms 1 |
| 2 | 7.528 | 1005 | 124-13-0 | Octanal | 128 | ms |
| 3 | 8.092 | 1018 | 5989-27-5 | d-Limonene | 136 | 23.2 |
| 4 | 9.132 | 1073 | 1195-32-0 | p-Cymenene | 132 | ms |
| 5 | 9.271 | 1082 | 78-70-6 | Linalool | 154 | 0.2 |
| 6 | 9.538 | 1206 | 99-48-9 | Carveol | 152 | ms |
| 7 | 9.716 | 1136 | 1845-30-3 | cis-Verbenol | 152 | 0.4 |
| 8 | 9.847 | 1088 | 6090-09-1 | Limona ketone | 138 | 0.1 |
| 9 | 9.966 | 1120 | 22771-44-4 | p-Mentha-2,8-dienol | 152 | 0.5 |
| 10 | 10.683 | 1174 | 141-27-5 | Citral | 152 | 0.2 |
| 11 | 10.993 | 1179 | 5948-04-9 | trans-Dihydrocarvone | 152 | 1.3 |
| 12 | 11.108 | 1179 | 3792-53-8 | cis-Dihydrocarvone | 152 | 0.5 |
| 13 | 11.340 | 1031 | 6909-30-4 | trans-Limonene oxide | 152 | 0.4 |
| 14 | 11.483 | 1206 | 1197-07-5 | trans-Carveol | 152 | 1.7 |
| 15 | 11.913 | 1190 | 2244-16-8 | d-Carvone | 150 | 66.5 |
| 16 | 12.056 | 1202 | 39903-97-4 | trans-Carvone oxide | 166 | 0.1 |
| 17 | 12.243 | 1207 | 2111-75-3 | Perilla aldehyde | 150 | 0.9 |
| 18 | 12.381 | 1335 | 57576-09-7 | Isopulegol acetate | 196 | 0.1 |
| 19 | 13.300 | 1031 | 1195-92-2 | cis-Limonene 1,2-epoxide | 152 | 2.0 |
| 20 | 13.409 | 1346 | 1946-00-5 | Limonene-1,2-diol | 170 | 0.1 |
| 21 | 13.655 | 1190 | 104-46-1 | Anethole | 148 | 0.2 |
| 22 | 13.826 | 1097 | 78-59-1 | Isophorone | 138 | 0.1 |
| 23 | 15.267 | 1278 | 499-75-2 | Carvacrol | 150 | 0.1 |
| 24 | 16.660 | 1507 | 1139-30-6 | Caryophyllene oxide | 220 | 0.2 |
| 25 | 16.780 | 1266 | 97-41-6 | Ethyl chrysanthemate | 196 | 0.1 |
| 26 | 17.540 | 1386 | 489-39-4 | Alloaromadendrene | 204 | 0.1 |
| 27 | 17.710 | 1507 | 1139-30-6 | Caryophyllene oxide | 220 | ms |
| 28 | 19.491 | 1754 | 502-69-2 | 2-Pentadecanone, 6,10,14-trimethyl- | 268 | 0.1 |
| Concentration (vol%) | Inhibition Zone Diameter (mm) | ||
|---|---|---|---|
| Staphylococcus aureus | Escherichia coli | ||
| EO from Carum carvi L. | 0.1 | 0 | 0 |
| 1.0 | 0 | 0 | |
| 10 | 6.4 ± 0.7 | 1.3 ± 0.1 | |
| 100 | 14.0 ± 2.3 | 4.3 ± 0.6 | |
| Sample | Antibacterial Activity (R) | |
|---|---|---|
| Staphylococcus aureus | Escherichia coli | |
| PP/TC | 0 | 0 |
| PP/TC/3EO | 0.7 | 0 |
| PP/TC/7EO | 1.4 | 0 |
| PP/TC/10EO | ≥5.3 | ≥6.4 |
| PP/TC/20EO | ≥5.3 | ≥6.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strasakova, M.; Pummerova, M.; Filatova, K.; Sedlarik, V. Immobilization of Caraway Essential Oil in a Polypropylene Matrix for Antimicrobial Modification of a Polymeric Surface. Polymers 2021, 13, 906. https://doi.org/10.3390/polym13060906
Strasakova M, Pummerova M, Filatova K, Sedlarik V. Immobilization of Caraway Essential Oil in a Polypropylene Matrix for Antimicrobial Modification of a Polymeric Surface. Polymers. 2021; 13(6):906. https://doi.org/10.3390/polym13060906
Chicago/Turabian StyleStrasakova, Monika, Martina Pummerova, Kateryna Filatova, and Vladimir Sedlarik. 2021. "Immobilization of Caraway Essential Oil in a Polypropylene Matrix for Antimicrobial Modification of a Polymeric Surface" Polymers 13, no. 6: 906. https://doi.org/10.3390/polym13060906
APA StyleStrasakova, M., Pummerova, M., Filatova, K., & Sedlarik, V. (2021). Immobilization of Caraway Essential Oil in a Polypropylene Matrix for Antimicrobial Modification of a Polymeric Surface. Polymers, 13(6), 906. https://doi.org/10.3390/polym13060906