Synthesis of Lignin-Based MMA-co-BA Hybrid Resins from Cornstalk Residue via RAFT Miniemulsion Polymerization and Their Characteristics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
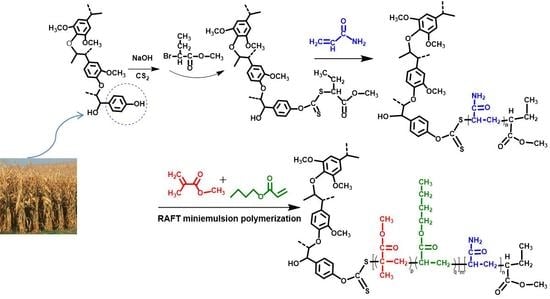
2.2. Synthesis of the Lignin-Based Polymeric RAFT Agent
2.3. Synthesis of the Lignin-Based MMA-co-BA Hybrid Resins
2.4. Analytics
2.4.1. Attenuated Total Reflectance–Fourier Transform Infrared (ATR-FTIR)
2.4.2. 1H Nuclear Magnetic Resonance (1H NMR)
2.4.3. Gel Permeation Chromatograph (GPC)
2.4.4. Transmission Electron Microscopy (TEM)
2.4.5. Differential Scanning Calorimetry (DSC)
2.4.6. Thermogravimetric Analysis (TGA)
2.4.7. Mechanical Performance
3. Results and Discussion
3.1. Characterization of Lignin-g-PAM
3.2. RAFT Miniemulsion Polymerization and Properties of the Lignin-Based MMA-co-BA Hybrid Resin
3.3. Characteristics of the Chemical Structure of the Lignin-Based Hybrid Acrylate Resins
3.4. Thermal Behavior
3.5. Mechanical Property
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Riess, G.; Schlienger, M.; Marti, S. New morphologies in rubber-modified polymers. J. Polym. Sci. Part B Polym. Phys. 1980, 17, 355–374. [Google Scholar] [CrossRef]
- Tong, J.D.; Jerome, R. Synthesis of poly(methyl methacrylate)-b-poly(n-butyl acrylate)-b-poly(methyl methacrylate) triblocks and their potential as thermoplastic elastomers. Polymer 2000, 41, 2499–2510. [Google Scholar] [CrossRef] [Green Version]
- Czech, Z. Crosslinking of pressure sensitive adhesive based on water-borne acrylate. Polym. Int. 2003, 52, 347–357. [Google Scholar] [CrossRef]
- Kaelble, D.H. Peel adhesion: Influence of surface energies and adhesive rheology. J. Adhesion. 1969, 1, 102–123. [Google Scholar] [CrossRef]
- Galià, M.; de Espinosa, L.M.; Ronda, J.C.; Lligadas, G.; Cádiz, V. Vegetable oil-based thermosetting polymers. Eur. J. Lipid Sci. Technol. 2010, 112, 87–96. [Google Scholar] [CrossRef]
- Lu, Y.-S.; Larock, R.C. New hybrid latexes from a soybean oil-based waterborne polyurethane and acrylics via emulsion polymerization. Biomacromolecules 2007, 8, 3108–3114. [Google Scholar] [CrossRef]
- Åkesson, D.; Skrifvars, M.; Walkenström, P. Preparation of thermoset composites from natural fibres and acrylate modified soybean oil resins. J. Appl. Polym. Sci. 2009, 114, 2502–2508. [Google Scholar] [CrossRef]
- Veith, C.; Diot-Néant, F.; Miller, S.A.; Allais, F. Synthesis and polymerization of bio-based acrylates: A review. Polym. Chem. 2020, 11, 7452–7470. [Google Scholar] [CrossRef]
- Ando, S.; Someya, Y.; Takahashi, T.; Shibata, M. Thermal and mechanical properties of photocured organic-inorganic hybrid nanocomposites of terpene-based acrylate resin and methacrylate-substituted polysilsesquioxane. J. Appl. Polym. Sci. 2010, 115, 3326–3331. [Google Scholar] [CrossRef]
- Winnacker, M.; Rieger, B. Recent progress in sustainable polymers obtained from cyclic terpenes: Synthesis, properties, and application potential. ChemSusChem 2015, 8, 2455–2471. [Google Scholar] [CrossRef] [PubMed]
- Sainz, M.F.; Souto, J.A.; Regentova, D.; Johansson, M.K.G.; Timhagen, S.T.; Irvine, D.J.; Buijsen, P.; Koning, C.E.; Stockman, R.A.; Howdle, S.M. A facile and green route to terpene derived acrylate and methacrylate monomers and simple free radical polymerisation to yield new renewable polymers and coatings. Polym. Chem. 2016, 7, 2882–2887. [Google Scholar] [CrossRef]
- Jahanshahi, S.; Pizzi, A.; Abdulkhani, A.; Shakeri, A. Analysis and testing of bisphenol A-free bio-based tannin epoxy-acrylic adhesives. Polymers 2016, 8, 143. [Google Scholar] [CrossRef] [Green Version]
- Bensabeh, N.; Moreno, A.; Roig, A.; Monaghan, O.R.; Ronda, J.C.; Cadiz, V.; Galia, M.; Howdle, S.M.; Lligadas, G.; Percec, V. Polyacrylates derived from biobased ethyl lactate solvent via SET-LRP. Biomacromolecules 2019, 20, 2135–2147. [Google Scholar] [CrossRef]
- Bensabeh, N.; Moreno, A.; Roig, A.; Rahimzadeh, M.; Rahimi, K.; Ronda, J.C.; Cádiz, V.; Galià, M.; Percec, V.; Rodriguez-Emmenegger, C.; et al. Photoinduced upgrading of lactic acid-based solvents to block copolymer surfactants. ACS Sustain. Chem. Eng. 2020, 8, 1276–1284. [Google Scholar] [CrossRef]
- Purushothaman, M.; Krishnan, P.S.G.; Nayak, S.K. Poly(alkyl lactate acrylate)s having tunable hydrophilicity. J. Appl. Polym. Sci. 2014, 131, 40962. [Google Scholar] [CrossRef]
- Anbinder, P.; Macchi, C.; Amalvy, J.; Somoza, A. Chitosan-graft-poly(n-butyl acrylate) copolymer: Synthesis and characterization of a natural/synthetic hybrid material. Carbohyd. Polym. 2016, 145, 86–94. [Google Scholar] [CrossRef]
- Cho, H.-G.; Park, S.-Y.; Jegal, J.; Song, B.-K.; Kim, H.-J. Preparation and characterization of acrylic polymers based on a novel acrylic monomer produced from vegetable oil. J. Appl. Polym. Sci. 2010, 116, 736–742. [Google Scholar] [CrossRef]
- De Espinosa, L.M.; Ronda, J.C.; Galià, M.; Cádiz, V. A new route to acrylate oils: Crosslinking and properties of acrylate triglycerides from high oleic sunflower oil. J. Polym. Sci. Part A: Polym. Chem. 2009, 47, 1159–1167. [Google Scholar] [CrossRef]
- Rengasamy, S.; Mannari, V. Development of soy-based UV-curable acrylate oligomers and study of their film properties. Prog. Org. Coat. 2013, 76, 78–85. [Google Scholar] [CrossRef]
- Garrison, T.F.; Murawski, A.; Quirino, R.L. Bio-based polymers with potential for biodegradability. Polymers 2016, 8, 262. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, X.-P.; Zhu, J.-J.; Liu, X.-Y.; Wang, Z.; Yan, J.-L. UV-curable coatings from multiarmed cardanol-based acrylate oligomers. ACS Sustain. Chem. Eng. 2015, 3, 1313–1320. [Google Scholar] [CrossRef]
- Hu, Y.; Shang, Q.-Q.; Tang, J.-J.; Wang, C.-N.; Zhang, F.; Jia, P.-Y.; Feng, G.-D.; Wu, Q.; Liu, C.-G.; Hu, L.-H.; et al. Use of cardanol-based acrylate as reactive diluent in UV-curable castor oil-based polyurethane acrylate resins. Ind. Crop. Prod. 2018, 117, 295–302. [Google Scholar] [CrossRef]
- Ladmiral, V.; Jeannin, R.; Fernandes Lizarazu, K.; Lai-Kee-Him, J.; Bron, P.; Lacroix-Desmazes, P.; Caillol, S. Aromatic biobased polymer latex from cardanol. Eur. Polym. J. 2017, 93, 785–794. [Google Scholar] [CrossRef]
- Liu, X.-H.; Wang, J.-F.; Li, S.-H.; Zhuang, X.-W.; Xu, Y.-Z.; Wang, C.-P.; Chu, F.-X. Preparation and properties of UV-absorbent lignin graft copolymer films from lignocellulosic butanol residue. Ind. Crop. Prod. 2014, 52, 633–641. [Google Scholar] [CrossRef]
- Yu, J.; Wang, J.-F.; Wang, C.-P.; Liu, Y.-P.; Xu, Y.-Z.; Tang, C.-B.; Chu, F.-X. UV-absorbent lignin-based multi-arm star thermoplastic elastomers. Macromol. Rapid Comm. 2015, 36, 398–404. [Google Scholar] [CrossRef]
- Bajwa, D.S.; Pourhashem, G.; Ullah, A.H.; Bajwa, S.G. A concise review of current lignin production, applications, products and their environmental impact. Ind. Crop. Prod. 2019, 139, 111526. [Google Scholar] [CrossRef]
- Jiang, X.; Abbati de Assis, C.; Kollman, M.; Sun, R.-K.; Jameel, H.; Chang, H.-M.; Gonzalez, R. Lignin fractionation from laboratory to commercialization: Chemistry, scalability and techno-economic analysis. Green Chem. 2020, 22, 7448–7459. [Google Scholar] [CrossRef]
- Sun, Z.; Fridrich, B.; de Santi, A.; Elangovan, S.; Barta, K. Bright side of lignin depolymerization: Toward new platform chemicals. Chem. Rev. 2018, 118, 614–678. [Google Scholar] [CrossRef] [Green Version]
- Sun, D.; Wang, B.; Wang, H.-M.; Li, M.-F.; Shi, Q.; Zheng, L.; Wang, S.-F.; Liu, S.-J.; Sun, R.-C. Structural elucidation of tobacco stalk lignin isolated by different integrated processes. Ind. Crop. Prod. 2019, 140, 111631. [Google Scholar] [CrossRef]
- Iravani, S.; Varma, R.S. Greener synthesis of lignin nanoparticles and their applications. Green Chem. 2020, 22, 612–636. [Google Scholar] [CrossRef]
- Li, R.J.-X.; Gutierrez, J.; Chung, Y.-L.; Frank, C.W.; Billington, S.L.; Sattely, E.S. A lignin-epoxy resin derived from biomass as an alternative to formaldehyde-based wood adhesives. Green Chem. 2018, 20, 1459–1466. [Google Scholar] [CrossRef]
- Bonini, C.; D’Auria, M.; Di Maggio, P.; Ferri, R. Characterization and degradation of lignin from steam explosion of pine and corn stalk of lignin: The role of superoxide ion and ozone. Ind. Crop. Prod. 2008, 27, 182–188. [Google Scholar] [CrossRef]
- Yang, Q.; Shi, J.; Lin, L.; Zhuang, J.; Pang, C.; Xie, T.; Liu, Y. Structural characterization of lignin in the process of cooking of cornstalk with solid alkali and active oxygen. J. Agric. Food Chem. 2012, 60, 4656–4661. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Chen, K.; Xu, J.; Li, J.; Mo, L. Bio-based polyurethane foam preparation employing lignin from corn stalk enzymatic hydrolysis residues. RSC Adv. 2018, 8, 15754–15761. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Yu, Y.; Di, M. Green modification of corn stalk lignin and preparation of environmentally friendly lignin-based wood adhesive. Polymers 2018, 10, 631. [Google Scholar] [CrossRef] [Green Version]
- Suckeveriene, R.Y.; Rahman, R.; Shtein, I.; Kharlamova, N.; Narkis, M. Synthesis of styrene-acrylamide copolymer by surfactant-free sonicated dynamic interfacial polymerization. Polym. Adv. Technol. 2012, 23, 1536–1542. [Google Scholar] [CrossRef]
- Friedman, M. Chemistry, biochemistry, and safety of acrylamide. A review. J. Agric. Food Chem. 2003, 51, 4504–4526. [Google Scholar] [CrossRef]
- Satoh, K. Controlled/living polymerization of renewable vinyl monomers into bio-based polymers. Polym. J. 2015, 47, 527–536. [Google Scholar] [CrossRef]
- Anand, V.; Agarwal, S.; Greiner, A.; Choudhary, V. Synthesis of methyl methacrylate andN-aryl itaconimide block copolymersvia atom-transfer radical polymerization. Polym. Int. 2005, 54, 823–828. [Google Scholar] [CrossRef]
- Satoh, K.; Lee, D.H.; Nagai, K.; Kamigaito, M. Precision synthesis of bio-based acrylic thermoplastic elastomer by RAFT polymerization of itaconic acid derivatives. Macromol. Rapid Comm. 2014, 35, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Colombani, D. Chain-growth control in free radical polymerization. Prog. Polym. Sci. 1997, 22, 1649–1720. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Xia, J.-H. Atom transfer radical polymerization. Chem. Rev. 2001, 101, 2921–2990. [Google Scholar] [CrossRef]
- Chiefari, J.; Chong, Y.K.B.; Ercole, F.; Krstina, J.; Jeffery, J.; Le, T.P.T.; Mayadunne, R.T.A.; Meijs, G.F.; Moad, C.L.; Moad, G.; et al. Living free-radical polymerization by reversible addition-fragmentation chain transfer: The RAFT process. Macromolecules 1998, 31, 5559–5562. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Tsarevsky, N.V. Macromolecular engineering by atom transfer radical polymerization. J. Am. Chem. Soc. 2014, 136, 6513–6533. [Google Scholar] [CrossRef] [PubMed]
- Chong, B.Y.K.; Le, T.P.T.; Moad, G.; Rizzardo, E.; Thang, S.H. A more versatile route to block copolymers and other polymers of complex architecture by living radical polymerization: The RAFT process. Macromolecules 1999, 32, 2071–2074. [Google Scholar] [CrossRef]
- Tong, J.D.; Moineau, G.; Leclere, P.; Bredas, J.L.; Lazzaroni, R.; Jerome, R. Synthesis, morphology, and mechanical properties of poly(methyl methacrylate)-b-poly(n-butyl acrylate)-b-poly(methyl methacrylate) triblocks. ligated anionic polymerization vs. atom transfer radical polymerization. Macromolecules 2000, 33, 470–479. [Google Scholar] [CrossRef]
- Shipp, D.A.; Wang, J.-L.; Matyjaszewski, K. Synthesis of acrylate and methacrylate block copolymers using atom transfer radical polymerization. Macromolecules 1998, 31, 8005–8008. [Google Scholar] [CrossRef]
- Moad, G.; Rizzardo, E.; Thang, S.H. Living radical polymerization by the RAFT process—A third update. Aust. J. Chem. 2012, 65, 985–1076. [Google Scholar] [CrossRef]
- Mayadunne, R.T.A.; Rizzardo, E.; Chiefari, J.; Chong, Y.K.; Moad, G.; Thang, S.H. Living radical polymerization with reversible addition-fragmentation chain transfer (RAFT polymerization) using dithiocarbamates as chain transfer agents. Macromolecules 1999, 32, 6977–6980. [Google Scholar] [CrossRef]
- Lowe, A.B.; McCormick, C.L. Reversible addition–fragmentation chain transfer (RAFT) radical polymerization and the synthesis of water-soluble (co)polymers under homogeneous conditions in organic and aqueous media. Prog. Polym. Sci. 2007, 32, 283–351. [Google Scholar] [CrossRef]
- Boyer, C.; Bulmus, V.; Davis, T.P.; Ladmiral, V.; Liu, J.; Perrier, S.B. Bioapplications of RAFT polymerization. Chem. Rev. 2009, 109, 5402–5436. [Google Scholar] [CrossRef] [PubMed]
- Moad, G.; Chong, Y.K.; Postma, A.; Rizzardo, E.; Thang, S.H. Advances in RAFT polymerization: The synthesis of polymers with defined end-groups. Polymer 2005, 46, 8458–8468. [Google Scholar] [CrossRef]
- Perrier, S.B.; Barner-Kowollik, C.; Quinn, J.F.; Vana, P.; Davis, T.P. Origin of inhibition effects in the reversible addition fragmentation chain transfer (RAFT) polymerization of methyl acrylate. Macromolecules 2002, 35, 8300–8306. [Google Scholar] [CrossRef]
- Nguyen, M.N.; Bressy, C.; Margaillan, A. Synthesis of novel random and block copolymers of tert-butyldimethylsilyl methacrylate and methyl methacrylate by RAFT polymerization. Polymer 2009, 50, 3086–3094. [Google Scholar] [CrossRef]
- Jiang, Y.-J.; Li, L.; Liu, J.-P.; Wang, R.; Wang, H.-S.; Tian, Q.; Li, X.-Y. Hydrophobic films of acrylic emulsion by incorporation of fluorine-based copolymer prepared through the RAFT emulsion copolymerization. J. Fluorine Chem. 2016, 183, 82–91. [Google Scholar] [CrossRef]
- Fu, L.-Y.; Yang, L.-T.; Dai, C.-L.; Zhao, C.-S.; Ma, L.-J. Thermal and mechanical properties of acrylated expoxidized-soybean oil-based thermosets. J. Appl. Polym. Sci. 2010, 117, 2220–2225. [Google Scholar] [CrossRef]
- De Oliveira, H.F.; Felisberti, M.I. Amphiphilic copolymers of sucrose methacrylate and acrylic monomers: Bio-based materials from renewable resource. Carbohyd. Polym. 2013, 94, 317–322. [Google Scholar] [CrossRef]
- Wang, J.-F.; Yao, K.-J.; Korich, A.L.; Li, S.-G.; Ma, S.-G.; Ploehn, H.J.; Iovine, P.M.; Wang, C.-P.; Chu, F.-X.; Tang, C.-B. Combining renewable gum rosin and lignin: Towards hydrophobic polymer composites by controlled polymerization. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 3728–3738. [Google Scholar] [CrossRef]
- Ferguson, C.J.; Hughes, R.J.; Nguyen, D.; Pham, B.T.T.; Gilbert, R.G.; Serelis, A.K.; Such, C.H.; Hawkett, B.S. Ab initio emulsion polymerization by RAFT-controlled self-assembly. Macromolecules 2005, 38, 2191–2204. [Google Scholar] [CrossRef]
- Rieger, J.; Osterwinter, G.; Bui, C.; Stoffelbach, F.O.; Charleux, B. Surfactant-free controlled/living radical emulsion (co)polymerization of n-butyl acrylate and methyl methacrylate via RAFT using amphiphilic poly(ethylene oxide)-based trithiocarbonate chain transfer agents. Macromolecules 2009, 42, 5518–5525. [Google Scholar] [CrossRef]
- Vosloo, J.J.; De Wet-Roos, D.; Tonge, M.P.; Sanderson, R.D. Controlled free radical polymerization in water-borne dispersion using reversible addition−fragmentation chain transfer. Macromolecules 2002, 35, 4894–4902. [Google Scholar] [CrossRef]
- Lansalot, M.; Davis, T.P.; Heuts, J.P.A. RAFT miniemulsion polymerization: Influence of the structure of the RAFT agent. Macromolecules 2002, 35, 7582–7591. [Google Scholar] [CrossRef]
- Morese-Seguela, B.; St-Jacques, M.; Renaud, J.M.; Prod’Homme, J. Microphase separation in low molecular weight styrene-isoprene diblock copolymers studied by DSC and 13 C NMR. Macromolecules 1980, 13, 100–106. [Google Scholar] [CrossRef]
- Xu, Y.-Z.; Yuan, L.; Wang, Z.-K.; Wilbon, P.A.; Wang, C.-P.; Chu, F.-X.; Tang, C.-B. Lignin and soy oil-derived polymeric biocomposites by “grafting from” RAFT polymerization. Green Chem. 2016, 18, 4974–4981. [Google Scholar] [CrossRef]
- Ferdosian, F.; Yuan, Z.-S.; Anderson, M.; Xu, C.-B. Synthesis and characterization of hydrolysis lignin-based epoxy resins. Ind. Crop. Prod. 2016, 91, 295–301. [Google Scholar] [CrossRef]








| Lignin-g-PAM Dosage (wt%) | Temperature (°C) | Conversion * (%) | Viscosity (mPa·s) | Mn (g/mol) | Mw/Mn | Gel Fraction (%) |
|---|---|---|---|---|---|---|
| 0 | 75 | 85.0 | 34.6 | 129,000 | 3.70 | 0.03 |
| 20 | 75 | 86.1 | 36.6 | 638,000 | 2.84 | 0.02 |
| 40 | 75 | 88.3 | 36.3 | 802,000 | 2.55 | 0.05 |
| 60 | 75 | 87.5 | 28.3 | 446,000 | 2.25 | 0.11 |
| 40 | 65 | 63.3 | 26.3 | 237,000 | 2.39 | 0.03 |
| 40 | 85 | 89.3 | 26.5 | 1,007,000 | 4.17 | 0.08 |
| Sample | Td 5% (°C) | Td 10% (°C) | Td max (°C) | Carbon Residue (%) |
|---|---|---|---|---|
| MMA-co-BA | 341 | 362 | 410 | 3.9 |
| 20% lignin-g-PAM | 242 | 358 | 403 | 9.8 |
| 40% lignin-g-PAM | 210 | 288 | 393 | 16.3 |
| 60% lignin-g-PAM | 188 | 273 | 390 | 18.0 |
| lignin | 163 | 240 | 327 | 44 |
| Dose of Lignin-g-PAM (wt%) | Failure Strain (%) | Tensile Strength (MPa) | Maximum Load (MPa) | Young’s Modulus (MPa) |
|---|---|---|---|---|
| 0 | 1216.50 | 2.60 | 2.36 | 0.27 |
| 20 | 863.96 | 6.56 | 8.90 | 17.03 |
| 40 | 419.62 | 8.53 | 24.01 | 198.78 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Li, N.; Wang, G.; Wang, C.; Chu, F. Synthesis of Lignin-Based MMA-co-BA Hybrid Resins from Cornstalk Residue via RAFT Miniemulsion Polymerization and Their Characteristics. Polymers 2021, 13, 968. https://doi.org/10.3390/polym13060968
Xu Y, Li N, Wang G, Wang C, Chu F. Synthesis of Lignin-Based MMA-co-BA Hybrid Resins from Cornstalk Residue via RAFT Miniemulsion Polymerization and Their Characteristics. Polymers. 2021; 13(6):968. https://doi.org/10.3390/polym13060968
Chicago/Turabian StyleXu, Yuzhi, Ning Li, Guangbin Wang, Chunpeng Wang, and Fuxiang Chu. 2021. "Synthesis of Lignin-Based MMA-co-BA Hybrid Resins from Cornstalk Residue via RAFT Miniemulsion Polymerization and Their Characteristics" Polymers 13, no. 6: 968. https://doi.org/10.3390/polym13060968
APA StyleXu, Y., Li, N., Wang, G., Wang, C., & Chu, F. (2021). Synthesis of Lignin-Based MMA-co-BA Hybrid Resins from Cornstalk Residue via RAFT Miniemulsion Polymerization and Their Characteristics. Polymers, 13(6), 968. https://doi.org/10.3390/polym13060968