Recent Advancements in Polymer/Liposome Assembly for Drug Delivery: From Surface Modifications to Hybrid Vesicles
Abstract
:1. Introduction
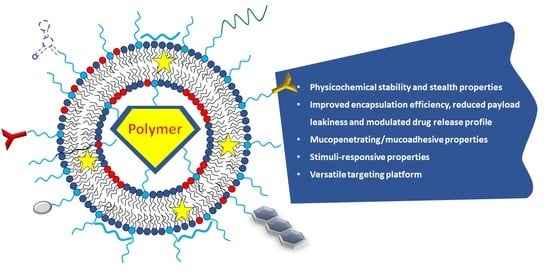
- Improving the physicochemical stability, the stealth properties, and the residence time in biological fluids
- Improving the encapsulation efficiency, reducing payload leakiness, and modulating drug release profile
- Conferring mucopenetrating/mucoadhesive properties
- Conferring stimuli-responsive properties
- Providing a versatile targeting platform
2. Polymer/Liposome Assembly to Improve Physicochemical Stability, Stealth Properties, and Residence Time of Vesicles in Biological Fluids
3. Polymer/Liposome Assembly to Improve Encapsulation Efficiency, Reducing Payload Leakiness and Modulating Drug Release Profile
4. Polymer/Liposome Assembly to Confer Mucopenetrating/Mucoadhesive Properties to Vesicles
5. Polymer/Liposome Assembly to Confer Stimuli-Responsive Properties to Vesicles
6. Polymer/Liposome Assembly to Provide Targeting Platform
7. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Walde, P. Preparation of Vesicles (Liposomes). In Encyclopedia of Nanoscience and Nanotechnology; Nalwa, H.S., Ed.; American Scientific Publishers: Valencia, CA, USA, 2003; Volume 8, pp. 1–37. [Google Scholar]
- Gregoriadis, G. Liposome Preparation and Related Techniques; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- New, R. Liposomes: A Practical Approach; IRL PRESS: New York, NY, USA; Oxford University Press: Oxford, UK, 1990. [Google Scholar]
- Stano, P.; D’Aguanno, E.; Bolz, J.; Fahr, A.; Luisi, P.L. A Remarkable Self-Organization Process as the Origin of Primitive Functional Cells. Angew. Chem. Int. Ed. 2013, 52, 13397–13400. [Google Scholar] [CrossRef] [PubMed]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bulbake, U.; Doppalapudi, S.; Kommineni, N.; Khan, W. Liposomal Formulations in Clinical Use: An Updated Review. Pharmaceutics 2017, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- De Leo, V.; Catucci, L.; Falqui, A.; Marotta, R.; Striccoli, M.; Agostiano, A.; Comparelli, R.; Milano, F. Hybrid Assemblies of Fluorescent Nanocrystals and Membrane Proteins in Liposomes. Langmuir 2014, 30, 1599–1608. [Google Scholar] [CrossRef] [PubMed]
- Depalo, N.; De Leo, V.; Corricelli, M.; Gristina, R.; Valente, G.; Casamassima, E.; Comparelli, R.; Laquintana, V.; Denora, N.; Fanizza, E.; et al. Lipid-based systems loaded with PbS nanocrystals: Near infrared emitting trackable nanovectors. J. Mater. Chem. B 2017, 5, 1471–1481. [Google Scholar] [CrossRef] [PubMed]
- De Leo, V.; Milano, F.; Paiano, A.; Bramato, R.; Giotta, L.; Comparelli, R.; Ruscigno, S.; Agostiano, A.; Bucci, C.; Catucci, L. Luminescent CdSe@ ZnS nanocrystals embedded in liposomes: A cytotoxicity study in HeLa cells. Toxicol. Res. 2017, 6, 947–957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J. Interfacing Zwitterionic Liposomes with Inorganic Nanomaterials: Surface Forces, Membrane Integrity, and Applications. Langmuir 2016, 32, 4393–4404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Leo, V.; Mattioli-Belmonte, M.; Cimmarusti, M.T.; Panniello, A.; Dicarlo, M.; Milano, F.; Agostiano, A.; De Giglio, E.; Catucci, L. Liposome-modified titanium surface: A strategy to locally deliver bioactive molecules. Colloids Surf. B Biointerfaces 2017, 158, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Scherphof, G.L.; Kamps, J.A.A.M. Liposome Opsonization. J. Liposome Res. 2005, 15, 109–139. [Google Scholar] [CrossRef] [PubMed]
- Rideau, E.; Dimova, R.; Schwille, P.; Wurm, F.R.; Landfester, K. Liposomes and polymersomes: A comparative review towards cell mimicking. Chem. Soc. Rev. 2018, 47, 8572–8610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dékány, G.; Csóka, I.; Erös, I. Interaction Between Liposomes and Neutral Polymers: Effect of Adsorption on Drug Release. J. Dispers. Sci. Technol. 2001, 22, 461–472. [Google Scholar] [CrossRef]
- Kozhikhova, K.V.; Ivantsova, M.N.; Tokareva, M.I.; Shulepov, I.D.; Tretiyakov, A.V.; Shaidarov, L.V.; Rusinov, V.L.; Mironov, M.A. Preparation of chitosan-coated liposomes as a novel carrier system for the antiviral drug Triazavirin. Pharm. Dev. Technol. 2018, 23, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Sybachin, A.V.; Lokova, A.Y.; Spiridonov, V.V.; Novoskol’tseva, O.A.; Shtykova, E.V.; Samoshin, V.V.; Migulin, V.A.; Yaroslavov, A.A. The Effect of Cationic Polylysine on the Release of an Encapsulated Substance from pH-Sensitive Anionic Liposomes. Polym. Sci. Ser. A 2019, 61, 308–316. [Google Scholar] [CrossRef]
- Hermal, F.; Frisch, B.; Specht, A.; Bourel-Bonnet, L.; Heurtault, B. Development and characterization of layer-by-layer coated liposomes with poly(L-lysine) and poly(L-glutamic acid) to increase their resistance in biological media. Int. J. Pharm. 2020, 586, 119568. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, A.K.; Gupta, S.; Thakur, J.; Gupta, S.; Pal, S.; Bajaj, A.; Srivastava, A. Polydopamine-on-liposomes: Stable nanoformulations, uniform coatings and superior antifouling performance. Nanoscale 2020, 12, 5021–5030. [Google Scholar] [CrossRef] [PubMed]
- De Leo, V.; Di Gioia, S.; Milano, F.; Fini, P.; Comparelli, R.; Mancini, E.; Agostiano, A.; Conese, M.; Catucci, L. Eudragit S100 Entrapped Liposome for Curcumin Delivery: Anti-Oxidative Effect in Caco-2 Cells. Coatings 2020, 10, 114. [Google Scholar] [CrossRef] [Green Version]
- De Leo, V.; Milano, F.; Mancini, E.; Comparelli, R.; Giotta, L.; Nacci, A.; Longobardi, F.; Garbetta, A.; Agostiano, A.; Catucci, L. Encapsulation of Curcumin-Loaded Liposomes for Colonic Drug Delivery in a pH-Responsive Polymer Cluster Using a pH-Driven and Organic Solvent-Free Process. Molecules 2018, 23, 739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shtenberg, Y.; Goldfeder, M.; Prinz, H.; Shainsky, J.; Ghantous, Y.; Abu El-Naaj, I.; Schroeder, A.; Bianco-Peled, H. Mucoadhesive alginate pastes with embedded liposomes for local oral drug delivery. Int. J. Biol. Macromol. 2018, 111, 62–69. [Google Scholar] [CrossRef] [PubMed]
- De Leo, V.; Ruscigno, S.; Trapani, A.; Di Gioia, S.; Milano, F.; Mandracchia, D.; Comparelli, R.; Castellani, S.; Agostiano, A.; Trapani, G.; et al. Preparation of drug-loaded small unilamellar liposomes and evaluation of their potential for the treatment of chronic respiratory diseases. Int. J. Pharm. 2018, 545, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Weber, C.; Voigt, M.; Simon, J.; Danner, A.-K.; Frey, H.; Mailänder, V.; Helm, M.; Morsbach, S.; Landfester, K. Functionalization of Liposomes with Hydrophilic Polymers Results in Macrophage Uptake Independent of the Protein Corona. Biomacromolecules 2019, 20, 2989–2999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Westen, R.v.d.; Hosta-Rigau, L.; Sutherland, D.S.; Goldie, K.N.; Albericio, F.; Postma, A.; Städler, B. Myoblast Cell Interaction with Polydopamine Coated Liposomes. Biointerphases 2012, 7, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, W.; Hu, C.M.; Fang, R.H.; Zhang, L. Liposome-like Nanostructures for Drug Delivery. J. Mater. Chem. B 2013, 1, 6569–6585. [Google Scholar] [CrossRef]
- Go, Y.K.; Kambar, N.; Leal, C. Hybrid Unilamellar Vesicles of Phospholipids and Block Copolymers with Crystalline Domains. Polymers 2020, 12, 1232. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.K.; Ho, J.C.S.; Roy, S.; Liedberg, B.; Nallani, M. Facile Mixing of Phospholipids Promotes Self-Assembly of Low-Molecular-Weight Biodegradable Block Co-Polymers into Functional Vesicular Architectures. Polymers 2020, 12, 979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dao, T.P.T.; Brûlet, A.; Fernandes, F.; Er-Rafik, M.; Ferji, K.; Schweins, R.; Chapel, J.P.; Fedorov, A.; Schmutz, M.; Prieto, M.; et al. Mixing Block Copolymers with Phospholipids at the Nanoscale: From Hybrid Polymer/Lipid Wormlike Micelles to Vesicles Presenting Lipid Nanodomains. Langmuir 2017, 33, 1705–1715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct. Target. Ther. 2018, 3, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gangadaran, P.; Hong, C.M.; Ahn, B.-C. An Update on in Vivo Imaging of Extracellular Vesicles as Drug Delivery Vehicles. Front. Pharmacol. 2018, 9, 169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beltrán-Gracia, E.; López-Camacho, A.; Higuera-Ciapara, I.; Velázquez-Fernández, J.B.; Vallejo-Cardona, A.A. Nanomedicine review: Clinical developments in liposomal applications. Cancer Nanotechnol. 2019, 10, 11. [Google Scholar] [CrossRef]
- Gubernator, J. Active methods of drug loading into liposomes: Recent strategies for stable drug entrapment and increased in vivo activity. Expert Opin. Drug Deliv. 2011, 8, 565–580. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, L.N.M.; Alcântara, A.C.S.; Rodrigues da Silva, G.H.; Franz-Montan, M.; Nista, S.V.G.; Castro, S.R.; Couto, V.M.; Guilherme, V.A.; de Paula, E. Advances in Hybrid Polymer-Based Materials for Sustained Drug Release. Int. J. Polym. Sci. 2017, 2017, 1231464. [Google Scholar] [CrossRef] [Green Version]
- Silvander, M.; Johnsson, M.; Edwards, K. Effects of PEG-lipids on permeability of phosphatidylcholine/cholesterol liposomes in buffer and in human serum. Chem. Phys. Lipids 1998, 97, 15–26. [Google Scholar] [CrossRef]
- Magarkar, A.; Róg, T.; Bunker, A. A computational study suggests that replacing PEG with PMOZ may increase exposure of hydrophobic targeting moiety. Eur. J. Pharm. Sci. 2017, 103, 128–135. [Google Scholar] [CrossRef]
- Riaz, M.K.; Riaz, M.A.; Zhang, X.; Lin, C.; Wong, K.H.; Chen, X.; Zhang, G.; Lu, A.; Yang, Z. Surface Functionalization and Targeting Strategies of Liposomes in Solid Tumor Therapy: A Review. Int. J. Mol. Sci. 2018, 19, 195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rissanen, S.; Wilkosz, N.; Bunker, A.; Jamróz, D.; Vattulainen, I.; Nowakowska, M.; Róg, T.; Kępczyński, M.; Dzieciuch-Rojek, M.; Kumorek, M. PEGylated liposomes as carriers of hydrophobic porphyrins. J. Phys. Chem. B 2015, 119, 6646–6657. [Google Scholar]
- Szebeni, J. Complement activation-related pseudoallergy caused by liposomes, micellar carriers of intravenous drugs, and radiocontrast agents. Crit. Rev. Ther. Drug Carr. Syst. 2001, 18, 567–606. [Google Scholar] [CrossRef]
- Szebeni, J.; Baranyi, L.; Savay, S.; Milosevits, J.; Bunger, R.; Laverman, P.; Metselaar, J.M.; Storm, G.; Chanan-Khan, A.; Liebes, L.; et al. Role of complement activation in hypersensitivity reactions to doxil and hynic PEG liposomes: Experimental and clinical studies. J. Liposome Res. 2002, 12, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Nag, O.K.; Awasthi, V. Surface Engineering of Liposomes for Stealth Behavior. Pharmaceutics 2013, 5, 542–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abu Lila, A.S.; Nawata, K.; Shimizu, T.; Ishida, T.; Kiwada, H. Use of polyglycerol (PG), instead of polyethylene glycol (PEG), prevents induction of the accelerated blood clearance phenomenon against long-circulating liposomes upon repeated administration. Int. J. Pharm. 2013, 456, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Whiteman, K.R.; Subr, V.; Ulbrich, K.; Torchilin, V.P. Poly(Hpma)-coated liposomes demonstrate prolonged circulation in mice. J. Liposome Res. 2001, 11, 153–164. [Google Scholar] [CrossRef]
- Takeuchi, H.; Kojima, H.; Yamamoto, H.; Kawashima, Y. Evaluation of circulation profiles of liposomes coated with hydrophilic polymers having different molecular weights in rats. J. Control. Release 2001, 75, 83–91. [Google Scholar] [CrossRef]
- Metselaar, J.M.; Bruin, P.; de Boer, L.W.T.; de Vringer, T.; Snel, C.; Oussoren, C.; Wauben, M.H.M.; Crommelin, D.J.A.; Storm, G.; Hennink, W.E. A Novel Family of l-Amino Acid-Based Biodegradable Polymer−Lipid Conjugates for the Development of Long-Circulating Liposomes with Effective Drug-Targeting Capacity. Bioconjugate Chem. 2003, 14, 1156–1164. [Google Scholar] [CrossRef] [PubMed]
- Levchenko, T.S.; Rammohan, R.; Lukyanov, A.N.; Whiteman, K.R.; Torchilin, V.P. Liposome clearance in mice: The effect of a separate and combined presence of surface charge and polymer coating. Int. J. Pharm. 2002, 240, 95–102. [Google Scholar] [CrossRef]
- Cao, Z.; Zhang, L.; Jiang, S. Superhydrophilic Zwitterionic Polymers Stabilize Liposomes. Langmuir 2012, 28, 11625–11632. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Kampf, N.; Goldberg, R.; Driver, M.J.; Klein, J. Poly-phosphocholinated Liposomes Form Stable Superlubrication Vectors. Langmuir 2019, 35, 6048–6054. [Google Scholar] [CrossRef] [PubMed]
- De Morais, F.A.P.; Gonçalves, R.S.; Braga, G.; Calori, I.R.; Pereira, P.C.S.; Batistela, V.R.; Caetano, W.; Hioka, N. Stable Dipalmitoylphosphatidylcholine Liposomes Coated with an F127 Copolymer for Hypericin Loading and Delivery. Acs Appl. Nano Mater. 2020, 3, 4530–4541. [Google Scholar] [CrossRef]
- Li, X.; Chen, D.; Le, C.; Zhu, C.; Gan, Y.; Hovgaard, L.; Yang, M. Novel mucus-penetrating liposomes as a potential oral drug delivery system: Preparation, in vitro characterization, and enhanced cellular uptake. Int. J. Nanomed. 2011, 6, 3151–3162. [Google Scholar]
- Ahn, H.; Park, J.-H. Liposomal delivery systems for intestinal lymphatic drug transport. Biomater. Res. 2016, 20, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatterjee, S.; Ooya, T. Copolymers Composed of 2-(Methacryloyloxy)ethyl Phosphorylcholine and Methacrylated Polyhedral Oligomeric Silsesquioxane as a Simple Modifier for Liposomes. ACS Appl. Polym. Mater. 2020, 2, 1909–1916. [Google Scholar] [CrossRef]
- Mineart, K.P.; Venkataraman, S.; Yang, Y.Y.; Hedrick, J.L.; Prabhu, V.M. Fabrication and Characterization of Hybrid Stealth Liposomes. Macromolecules 2018, 51, 3184–3192. [Google Scholar] [CrossRef]
- Ravar, F.; Saadat, E.; Gholami, M.; Dehghankelishadi, P.; Mahdavi, M.; Azami, S.; Dorkoosh, F.A. Hyaluronic acid-coated liposomes for targeted delivery of paclitaxel, in-vitro characterization and in-vivo evaluation. J. Control. Release 2016, 229, 10–22. [Google Scholar] [CrossRef]
- Liu, W.; Liu, J.; Liu, W.; Li, T.; Liu, C. Improved Physical and in Vitro Digestion Stability of a Polyelectrolyte Delivery System Based on Layer-by-Layer Self-Assembly Alginate–Chitosan-Coated Nanoliposomes. J. Agric. Food Chem. 2013, 61, 4133–4144. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Patil, S.R.; Swarnakar, N.K.; Agrawal, A.K. Oral Delivery of Doxorubicin Using Novel Polyelectrolyte-Stabilized Liposomes (Layersomes). Mol. Pharm. 2012, 9, 2626–2635. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Kumar, D.; Swarnakar, N.K.; Thanki, K. Polyelectrolyte stabilized multilayered liposomes for oral delivery of paclitaxel. Biomaterials 2012, 33, 6758–6768. [Google Scholar] [CrossRef]
- Barea, M.J.; Jenkins, M.J.; Lee, Y.S.; Johnson, P.; Bridson, R.H. Encapsulation of Liposomes within pH Responsive Microspheres for Oral Colonic Drug Delivery. Int. J. Biomater. 2012, 2012, 458712. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.X.; Huang, L.; Gauthier, M.; Yang, G.; Wang, Q. Recent advances in liposome surface modification for oral drug delivery. Nanomedicine 2016, 11, 1169–1185. [Google Scholar] [CrossRef] [PubMed]
- Manconi, M.; Nácher, A.; Merino, V.; Merino-Sanjuan, M.; Manca, M.L.; Mura, C.; Mura, S.; Fadda, A.M.; Diez-Sales, O. Improving Oral Bioavailability and Pharmacokinetics of Liposomal Metformin by Glycerolphosphate–Chitosan Microcomplexation. Aaps Pharmscitech. 2013, 14, 485–496. [Google Scholar] [CrossRef]
- Deygen, I.M.; Seidl, C.; Kölmel, D.K.; Bednarek, C.; Heissler, S.; Kudryashova, E.V.; Bräse, S.; Schepers, U. Novel Prodrug of Doxorubicin Modified by Stearoylspermine Encapsulated into PEG-Chitosan-Stabilized Liposomes. Langmuir 2016, 32, 10861–10869. [Google Scholar] [CrossRef] [PubMed]
- Odeniyi, M.A.; Omoteso, O.A.; Adepoju, A.O.; Jaiyeoba, K.T. Starch nanoparticles in drug delivery: A review. Polim. W Med. 2018, 48, 41–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nahar, K.; Absar, S.; Patel, B.; Ahsan, F. Starch-coated magnetic liposomes as an inhalable carrier for accumulation of fasudil in the pulmonary vasculature. Int. J. Pharm. 2014, 464, 185–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salem, H.F.; Kharshoum, R.M.; Mahmoud, M.; Azim, S.A.; Ebeid, E.-Z.M. Development and characterization of a novel nano-liposomal formulation of Alendronate Sodium loaded with biodegradable polymer. Ars Pharm. 2018, 59, 9–20. [Google Scholar]
- Hosny, K.M.; Ahmed, O.A.A.; Al-Abdali, R.T. Enteric-coated alendronate sodium nanoliposomes: A novel formula to overcome barriers for the treatment of osteoporosis. Expert Opin. Drug Deliv. 2013, 10, 741–746. [Google Scholar] [CrossRef]
- Chen, Y.; Xia, G.; Zhao, Z.; Xue, F.; Gu, Y.; Chen, C.; Zhang, Y. 7,8-Dihydroxyflavone nano-liposomes decorated by crosslinked and glycosylated lactoferrin: Storage stability, antioxidant activity, in vitro release, gastrointestinal digestion and transport in Caco-2 cell monolayers. J. Funct. Foods 2020, 65, 103742. [Google Scholar] [CrossRef]
- Wijetunge, S.S.; Wen, J.; Yeh, C.-K.; Sun, Y. Lectin-Conjugated Liposomes as Biocompatible, Bioadhesive Drug Carriers for the Management of Oral Ulcerative Lesions. ACS Appl. Bio. Mater. 2018, 1, 1487–1495. [Google Scholar] [CrossRef]
- Lopez, A.; Liu, J. DNA Oligonucleotide-Functionalized Liposomes: Bioconjugate Chemistry, Biointerfaces, and Applications. Langmuir 2018, 34, 15000–15013. [Google Scholar] [CrossRef] [PubMed]
- Baumann, K.N.; Piantanida, L.; García-Nafría, J.; Sobota, D.; Voïtchovsky, K.; Knowles, T.P.J.; Hernández-Ainsa, S. Coating and Stabilization of Liposomes by Clathrin-Inspired DNA Self-Assembly. Acs Nano 2020, 14, 2316–2323. [Google Scholar] [CrossRef] [PubMed]
- Almeida, B.; Nag, O.K.; Rogers, K.E.; Delehanty, J.B. Recent Progress in Bioconjugation Strategies for Liposome-Mediated Drug Delivery. Molecules 2020, 25, 5672. [Google Scholar] [CrossRef] [PubMed]
- Grit, M.; Crommelin, D.J.A. Chemical stability of liposomes: Implications for their physical stability. Chem. Phys. Lipids 1993, 64, 3–18. [Google Scholar] [CrossRef]
- Khan, A.A.; Allemailem, K.S.; Almatroodi, S.A.; Almatroudi, A.; Rahmani, A.H. Recent strategies towards the surface modification of liposomes: An innovative approach for different clinical applications. 3 Biotech 2020, 10, 163. [Google Scholar] [CrossRef]
- Zarrabi, A.; Zarepour, A.; Khosravi, A.; Alimohammadi, Z.; Thakur, V.K. Synthesis of Curcumin Loaded Smart pH-Responsive Stealth Liposome as a Novel Nanocarrier for Cancer Treatment. Fibers 2021, 9, 19. [Google Scholar] [CrossRef]
- Charoensit, P.; Pompimon, W.; Khorana, N.; Sungthongjeen, S. Effect of amide linkage of PEG-lipid conjugates on the stability and cytotoxic activity of goniodiol loaded in PEGylated liposomes. J. Drug Deliv. Sci. Technol. 2019, 50, 1–8. [Google Scholar] [CrossRef]
- Hashizaki, K.; Taguchi, H.; Itoh, C.; Sakai, H.; Abe, M.; Saito, Y.; Ogawa, N. Effects of Poly(ethylene glycol) (PEG) Concentration on the Permeability of PEG-Grafted Liposomes. Chem. Pharm. Bull. 2005, 53, 27–31. [Google Scholar] [CrossRef] [Green Version]
- Caddeo, C.; Díez-Sales, O.; Pons, R.; Carbone, C.; Ennas, G.; Puglisi, G.; Fadda, A.M.; Manconi, M. Cross-linked chitosan/liposome hybrid system for the intestinal delivery of quercetin. J. Colloid Interface Sci. 2016, 461, 69–78. [Google Scholar] [CrossRef] [Green Version]
- Abu Lila, A.S.; Ishida, T. Liposomal Delivery Systems: Design Optimization and Current Applications. Biol. Pharm. Bull. 2017, 40, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blume, G.; Cevc, G. Liposomes for the sustained drug release in vivo. Biochim. Et Biophys. Acta 1990, 1029, 91–97. [Google Scholar] [CrossRef]
- Allen, T.M.; Hansen, C.; Rutledge, J. Liposomes with prolonged circulation times: Factors affecting uptake by reticuloendothelial and other tissues. Biochim. Et Biophys. Acta 1989, 981, 27–35. [Google Scholar] [CrossRef]
- Chen, E.; Chen, B.-M.; Su, Y.-C.; Chang, Y.-C.; Cheng, T.-L.; Barenholz, Y.; Roffler, S.R. Premature Drug Release from Polyethylene Glycol (PEG)-Coated Liposomal Doxorubicin via Formation of the Membrane Attack Complex. ACS Nano 2020, 14, 7808–7822. [Google Scholar] [CrossRef] [PubMed]
- Mufamadi, M.S.; Pillay, V.; Choonara, Y.E.; Du Toit, L.C.; Modi, G.; Naidoo, D.; Ndesendo, V.M.K. A Review on Composite Liposomal Technologies for Specialized Drug Delivery. J. Drug Deliv. 2011, 2011, 939851. [Google Scholar] [CrossRef] [PubMed]
- Ciobanu, B.C.; Cadinoiu, A.N.; Popa, M.; Desbrières, J.; Peptu, C.A. Modulated release from liposomes entrapped in chitosan/gelatin hydrogels. Mater. Sci. Eng. C 2014, 43, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Yan, Y.; Liu, H.; Chen, H.; Pan, G.; Deng, L.; Cui, W. Mechanically enhanced lipo-hydrogel with controlled release of multi-type drugs for bone regeneration. Appl. Mater. Today 2018, 12, 294–308. [Google Scholar] [CrossRef]
- Bansil, R.; Turner, B.S. The biology of mucus: Composition, synthesis and organization. Adv. Drug Deliv. Rev. 2018, 124, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Netsomboon, K.; Bernkop-Schnürch, A. Mucoadhesive vs. mucopenetrating particulate drug delivery. Eur. J. Pharm. Biopharm. 2016, 98, 76–89. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Lu, Y.; Qi, J.; Zhu, Q.; Chen, Z.; Wu, W. Adapting liposomes for oral drug delivery. Acta Pharm. Sin. B 2019, 9, 36–48. [Google Scholar] [CrossRef]
- Takeuchi, H.; Matsui, Y.; Yamamoto, H.; Kawashima, Y. Mucoadhesive properties of carbopol or chitosan-coated liposomes and their effectiveness in the oral administration of calcitonin to rats. J. Control Release 2003, 86, 235–242. [Google Scholar] [CrossRef]
- Jung, I.-W.; Han, H.-K. Effective mucoadhesive liposomal delivery system for risedronate: Preparation and in vitro/in vivo characterization. Int. J. Nanomed. 2014, 9, 2299–2306. [Google Scholar]
- Abdellatif, M.; Khalil, I.; Elakkad, Y.; Eliwa, H.; Samir, T.; Al-Mokaddem, A. Formulation and Characterization of Sertaconazole Nitrate Mucoadhesive Liposomes for Vaginal Candidiasis. Int. J. Nanomed. 2020, 15, 4079–4090. [Google Scholar] [CrossRef]
- Karn, P.R.; Vanić, Z.; Pepić, I.; Skalko-Basnet, N. Mucoadhesive liposomal delivery systems: The choice of coating material. Drug Dev. Ind. Pharm. 2011, 37, 482–488. [Google Scholar] [CrossRef] [Green Version]
- Smart, J.D. The basics and underlying mechanisms of mucoadhesion. Adv. Drug Deliv. Rev. 2005, 57, 1556–1568. [Google Scholar] [CrossRef] [PubMed]
- Adamczak, M.I.; Hagesaether, E.; Smistad, G.; Hiorth, M. An in vitro study of mucoadhesion and biocompatibility of polymer coated liposomes on HT29-MTX mucus-producing cells. Int. J. Pharm. 2016, 498, 225–233. [Google Scholar] [CrossRef]
- Jøraholmen, M.W.; Škalko-Basnet, N.; Acharya, G.; Basnet, P. Resveratrol-loaded liposomes for topical treatment of the vaginal inflammation and infections. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2015, 79, 112–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berginc, K.; Suljaković, S.; Škalko-Basnet, N.; Kristl, A. Mucoadhesive liposomes as new formulation for vaginal delivery of curcumin. Eur. J. Pharm. Biopharm. Off. J. Arb. Fur Pharm. Verfahr. E.V 2014, 87, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Federer, C.; Kurpiers, M.; Bernkop-Schnürch, A. Thiolated Chitosans: A Multi-talented Class of Polymers for Various Applications. Biomacromolecules 2021, 22, 24–56. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Lee, S.H.; Song, J.G.; Kim, H.Y.; Han, H.-K. Enhanced oral absorption of sorafenib via the layer-by-layer deposition of a pH-sensitive polymer and glycol chitosan on the liposome. Int. J. Pharm. 2018, 544, 14–20. [Google Scholar] [CrossRef]
- Al Harthi, S.; Alavi, S.E.; Radwan, M.A.; El Khatib, M.M.; AlSarra, I.A. Nasal delivery of donepezil HCl-loaded hydrogels for the treatment of Alzheimer’s disease. Sci. Rep. 2019, 9, 9563. [Google Scholar] [CrossRef]
- Kaldybekov, D.B.; Tonglairoum, P.; Opanasopit, P.; Khutoryanskiy, V.V. Mucoadhesive maleimide-functionalised liposomes for drug delivery to urinary bladder. Eur. J. Pharm. Sci. 2018, 111, 83–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jøraholmen, M.W.; Basnet, P.; Acharya, G.; Škalko-Basnet, N. PEGylated liposomes for topical vaginal therapy improve delivery of interferon alpha. Eur. J. Pharm. Biopharm. Off. J. Arb. Fur Pharm. Verfahr. E.V 2017, 113, 132–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Q.; Ensign, L.M.; Boylan, N.J.; Schön, A.; Gong, X.; Yang, J.C.; Lamb, N.W.; Cai, S.; Yu, T.; Freire, E.; et al. Impact of Surface Polyethylene Glycol (PEG) Density on Biodegradable Nanoparticle Transport in Mucus ex Vivo and Distribution in Vivo. ACS Nano 2015, 9, 9217–9227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamazoe, E.; Fang, J.-Y.; Tahara, K. Oral mucus-penetrating PEGylated liposomes to improve drug absorption: Differences in the interaction mechanisms of a mucoadhesive liposome. Int. J. Pharm. 2021, 593, 120148. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, T.; Wei, S.; Zhou, C.; Lan, Y.; Cao, A.; Yang, J.; Wang, W. Mucus adhesion- and penetration-enhanced liposomes for paclitaxel oral delivery. Int. J. Pharm. 2018, 537, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Wike-Hooley, J.L.; Haveman, J.; Reinhold, H.S. The relevance of tumour pH to the treatment of malignant disease. Radiother. Oncol. 1984, 2, 343–366. [Google Scholar] [CrossRef]
- Yang, Y.L.; Liu, D.W.; Wang, X.T.; Long, Y.; Zhou, X.; Chai, W.Z. Body temperature control in patients with refractory septic shock: Too much may be harmful. Chin. Med. J. 2013, 126, 1809–1813. [Google Scholar] [PubMed]
- Kato, Y.; Ozawa, S.; Miyamoto, C.; Maehata, Y.; Suzuki, A.; Maeda, T.; Baba, Y. Acidic extracellular microenvironment and cancer. Cancer Cell Int. 2013, 13, 89. [Google Scholar] [CrossRef] [Green Version]
- Drummond, D.C.; Zignani, M.; Leroux, J. Current status of pH-sensitive liposomes in drug delivery. Prog. Lipid Res. 2000, 39, 409–460. [Google Scholar] [CrossRef]
- Soares, D.C.; de Oliveira, M.C.; de Barros, A.L.; Cardoso, V.N.; Ramaldes, G.A. Liposomes radiolabeled with (159)Gd: In vitro antitumoral activity, biodistribution study and scintigraphic image in Ehrlich tumor bearing mice. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2011, 43, 290–296. [Google Scholar]
- Connor, J.; Norley, N.; Huang, L. Biodistribution of pH-sensitive immunoliposomes. Biochim. Et Biophys. Acta 1986, 884, 474–481. [Google Scholar] [CrossRef]
- Chen, T.; Choi, L.S.; Einstein, S.; Klippenstein, M.A.; Scherrer, P.; Cuhis, P.R. Proton-Induced Permeability and Fusion of Large Unilamellar Vesicles by Covalently Conjugated Poly(2-Ethylacrylic Acid). J. Liposome Res. 1999, 9, 387–405. [Google Scholar] [CrossRef]
- Roux, E.; Passirani, C.; Scheffold, S.; Benoit, J.-P.; Leroux, J.-C. Serum-stable and long-circulating, PEGylated, pH-sensitive liposomes. J. Control. Release 2004, 94, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Zignani, M.; Drummond, D.C.; Meyer, O.; Hong, K.; Leroux, J.C. In vitro characterization of a novel polymeric-based pH-sensitive liposome system. Biochim. Et Biophys. Acta 2000, 1463, 383–394. [Google Scholar] [CrossRef] [Green Version]
- Ghanbarzadeh, S.; Arami, S.; Pourmoazzen, Z.; Khorrami, A. Improvement of the antiproliferative effect of rapamycin on tumor cell lines by poly (monomethylitaconate)-based pH-sensitive, plasma stable liposomes. Colloids Surf. B Biointerfaces 2014, 115, 323–330. [Google Scholar] [CrossRef]
- Kono, K.; Kaiden, T.; Yuba, E.; Sakanishi, Y.; Harada, A. Synthesis of oligo(ethylene glycol)-modified hyperbranched poly(glycidol)s for dual sensitization of liposomes to pH and temperature. J. Taiwan Inst. Chem. Eng. 2014, 45, 3054–3061. [Google Scholar] [CrossRef]
- Massey, J.B. Effect of cholesteryl hemisuccinate on the interfacial properties of phosphatidylcholine bilayers. Biochim. Et Biophys. Acta 1998, 1415, 193–204. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Paxton, J.W.; Wu, Z. Enhanced pH-Responsiveness, Cellular Trafficking, Cytotoxicity and Long-circulation of PEGylated Liposomes with Post-insertion Technique Using Gemcitabine as a Model Drug. Pharm. Res. 2015, 32, 2428–2438. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Hu, M.; Yu, X.; Li, Y.; Fu, Y.; Zhou, X.; Zhang, D.; Li, J. Design and evaluation of pH-sensitive liposomes constructed by poly(2-ethyl-2-oxazoline)-cholesterol hemisuccinate for doxorubicin delivery. Eur. J. Pharm. Biopharm. 2015, 91, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zhang, W.; Li, Y.; Ye, F.F.; Yin, P.P.; Yu, X.; Hu, M.N.; Fu, Y.S.; Wang, C.; Shang, D.J. The Bifunctional Liposomes Constructed by Poly(2-ethyl-oxazoline)-cholesteryl Methyl Carbonate: An Effectual Approach to Enhance Liposomal Circulation Time, pH-Sensitivity and Endosomal Escape. Pharm. Res. 2014, 31, 3038–3050. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-M.; Lee, O.-S.; O’Halloran, T.V.; Schatz, G.C.; Nguyen, S.T. Triggered Release of Pharmacophores from [Ni(HAsO3)]-Loaded Polymer-Caged Nanobin Enhances Pro-apoptotic Activity: A Combined Experimental and Theoretical Study. Acs Nano 2011, 5, 3961–3969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jelezova, I.; Drakalska, E.; Momekova, D.; Shalimova, N.; Momekov, G.; Konstantinov, S.; Rangelov, S.; Pispas, S. Curcumin loaded pH-sensitive hybrid lipid/block copolymer nanosized drug delivery systems. Eur. J. Pharm. Sci. 2015, 78, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-F.; Chiang, W.-H.; Tsai, P.-L.; Chern, C.-S.; Chiu, H.-C. Novel hybrid vesicles co-assembled from a cationic lipid and PAAc-g-mPEG with pH-triggered transmembrane channels for controlled drug release. Chem. Commun. 2011, 47, 10978–10980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyazaki, M.; Yuba, E.; Hayashi, H.; Harada, A.; Kono, K. Hyaluronic Acid-Based pH-Sensitive Polymer-Modified Liposomes for Cell-Specific Intracellular Drug Delivery Systems. Bioconjugate Chem. 2018, 29, 44–55. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.W.; Niidome, T.; Lee, R. Glycol Chitosan-Docosahexaenoic Acid Liposomes for Drug Delivery: Synergistic Effect of Doxorubicin-Rapamycin in Drug-Resistant Breast Cancer. Mar. Drugs 2019, 17, 581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Cai, F.; Shen, X.; Su, H. A high stable pH-temperature dual-sensitive liposome for tuning anticancer drug release. Synth. Syst. Biotechnol. 2020, 5, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Rukavina, Z.; Vanić, Ž. Current Trends in Development of Liposomes for Targeting Bacterial Biofilms. Pharmaceutics 2016, 8, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, R.; Mai, Y.; Zhao, Y.; Hou, Y.; Liu, Y.; Yang, J. Targeting strategies of liposomal subunit vaccine delivery systems to improve vaccine efficacy. J. Drug Target. 2019, 27, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; van der Meel, R.; Chen, X.; Lammers, T. The EPR effect and beyond: Strategies to improve tumor targeting and cancer nanomedicine treatment efficacy. Theranostics 2020, 10, 7921–7924. [Google Scholar] [CrossRef] [PubMed]
- Yingchoncharoen, P.; Kalinowski, D.S.; Richardson, D.R. Lipid-Based Drug Delivery Systems in Cancer Therapy: What Is Available and What Is Yet to Come. Pharmacol. Rev. 2016, 68, 701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maranhão, R.C.; Vital, C.G.; Tavoni, T.M.; Graziani, S.R. Clinical experience with drug delivery systems as tools to decrease the toxicity of anticancer chemotherapeutic agents. Expert Opin. Drug Deliv. 2017, 14, 1217–1226. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhan, P.; De Clercq, E.; Lou, H.; Liu, X. Current drug research on PEGylation with small molecular agents. Prog. Polym. Sci. 2013, 38, 421–444. [Google Scholar] [CrossRef]
- Golombek, S.K.; May, J.-N.; Theek, B.; Appold, L.; Drude, N.; Kiessling, F.; Lammers, T. Tumor targeting via EPR: Strategies to enhance patient responses. Adv. Drug Deliv. Rev. 2018, 130, 17–38. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V.P. Recent approaches to intracellular delivery of drugs and DNA and organelle targeting. Annu. Rev. Biomed. Eng. 2006, 8, 343–375. [Google Scholar] [CrossRef]
- Moreira, J.N.; Ishida, T.; Gaspar, R.; Allen, T.M. Use of the post-insertion technique to insert peptide ligands into pre-formed stealth liposomes with retention of binding activity and cytotoxicity. Pharm. Res. 2002, 19, 265–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, W.; Leung, S.S.; To, K.K. Updates on the use of liposomes for active tumor targeting in cancer therapy. Nanomedicine 2020, 15, 303–318. [Google Scholar] [CrossRef] [PubMed]
- Cadinoiu, A.N.; Rata, D.M.; Atanase, L.I.; Daraba, O.M.; Gherghel, D.; Vochita, G.; Popa, M. Aptamer-Functionalized Liposomes as a Potential Treatment for Basal Cell Carcinoma. Polymers 2019, 11, 1515. [Google Scholar] [CrossRef] [PubMed] [Green Version]












Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Leo, V.; Milano, F.; Agostiano, A.; Catucci, L. Recent Advancements in Polymer/Liposome Assembly for Drug Delivery: From Surface Modifications to Hybrid Vesicles. Polymers 2021, 13, 1027. https://doi.org/10.3390/polym13071027
De Leo V, Milano F, Agostiano A, Catucci L. Recent Advancements in Polymer/Liposome Assembly for Drug Delivery: From Surface Modifications to Hybrid Vesicles. Polymers. 2021; 13(7):1027. https://doi.org/10.3390/polym13071027
Chicago/Turabian StyleDe Leo, Vincenzo, Francesco Milano, Angela Agostiano, and Lucia Catucci. 2021. "Recent Advancements in Polymer/Liposome Assembly for Drug Delivery: From Surface Modifications to Hybrid Vesicles" Polymers 13, no. 7: 1027. https://doi.org/10.3390/polym13071027
APA StyleDe Leo, V., Milano, F., Agostiano, A., & Catucci, L. (2021). Recent Advancements in Polymer/Liposome Assembly for Drug Delivery: From Surface Modifications to Hybrid Vesicles. Polymers, 13(7), 1027. https://doi.org/10.3390/polym13071027