Molecular Mobility in Oriented and Unoriented Membranes Based on Poly[2-(Aziridin-1-yl)ethanol]
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization Techniques
3. Results
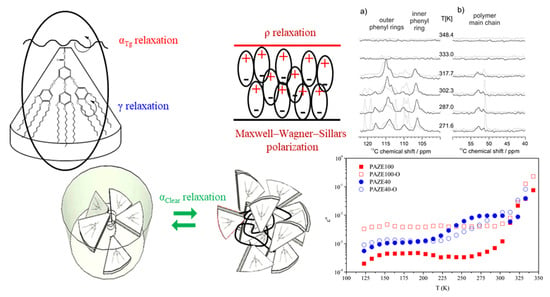
3.1. Calorimetric, X-ray and NMR Analyses
3.2. Dielectric Response of PAZE Polymer and Copolymers
3.3. Macromolecular Cooperativeness and Thermal Activation of Dielectric Relaxations
- The γ relaxation seems to depend only on the benzyloxy terminal group of the mesogenic side groups. It seems to be affected by the orientation of the membrane, especially for the PAZE100: this may be due to the tendency to crystallize this polymer when submitted to annealing.
- The two relaxations αTg and αClear, which contribute to the dielectric α relaxation zone, have different behaviour depending on the mesogenic substitution degree. The orientation during preparation did not have an effect on the temperature of αClear relaxation. The values of αClear temperature peak at 1 kHz are: 333 K and 330 K for PAZE40 and 337 K for both unoriented and oriented PAZE100 membranes, respectively, probably because the possible crystallization or orientation of the side chains could have already disappeared during the melting, which temperature is lower than the clearing process. On the other hand, the orientation has a perceptible effect on the values of αTg temperature peak at 1 kHz, especially for the PAZE40, which values increase from 268 K to 320 K for the unoriented and oriented membranes, respectively. This effect is minor in the PAZE 100 which values are 263 K and 270 K, respectively. Unfortunately, this behaviour could not be checked by calorimetric analysis since the glass transition was not visible.
3.3.1. Molecular Origin of the γ Relaxation Zone
3.3.2. Macromolecular Origin of the α Relaxation Zone
- Molecular precursor motions of the melting of a certain crystalline portion of the lateral dendritic chains;
- The associated motion to the glass transition;
- Molecular precursor motions of the clearing transition. However, only two dielectric relaxations can be clearly distinguished: one with lower intensity ascribed to the glass transition, i.e., αTg, because the percentage of crystallinity is indeed very small, as shown by DSC and XRD results, and should be overlapped by the glass transition processes; the other one much more prominent related to clearing transition, i.e., αClear.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Birshtein, T.M.; Amoskov, V.M.; Smirnov, E.O. Theory of Nematic Ordering in Supramolecular Systems: Self-Assembly of Mesogenic Groups and Self-Organization of the System. Polym. Sci. Ser. A 2016, 58, 593–605. [Google Scholar] [CrossRef]
- Dulyea, L.M.; Fyles, T.M.; Robertson, G.D. Coupled Transport Membranes Incorporating a Polymeric Crown Ether Carboxylic Acid. J. Membr. Sci. 1987, 34, 87–108. [Google Scholar] [CrossRef]
- Every, H.A.; Mendes, E.; Picken, S.J. Ordered Structures in Proton Conducting Membranes from Supra-molecular Liquid Crystal Polymers. J. Phys. Chem. B 2006, 110, 23729–23735. [Google Scholar] [CrossRef]
- McHattie, G.S.; Imrie, C.T.; Ingram, M.D. Ionically Conducting Side Chain Liquid Crystal Polymer Electrolytes. Electrochim. Acta 1998, 43, 1151–1154. [Google Scholar] [CrossRef]
- Shibaev, V.P. Liquid-Crystalline Polymers: Past, Present, and Future. Polym. Sci. Ser. A 2009, 51, 1131–1193. [Google Scholar] [CrossRef]
- Bogdanowicz, K.A.; Bhosale, S.V.; Li, Y.; Vankelecom, I.F.J.; Garcia-Valls, R.; Reina, J.A.; Giamberini, M. Mimicking Nature: Biomimetic Ionic Channels. J. Membr. Sci. 2016, 509, 10–18. [Google Scholar] [CrossRef]
- Bogdanowicz, K.A.; Rapsilber, G.A.; Reina, J.A.; Giamberini, M. Liquid Crystalline Polymeric Wires for Selective Proton Transport, Part 1: Wires Preparation. Polymer 2016, 92, 50–57. [Google Scholar] [CrossRef]
- Bogdanowicz, K.A.; Sistat, P.; Reina, J.A.; Giamberini, M. Liquid Crystalline Polymeric Wires for Selec-tive Proton Transport, Part 2: Ion Transport in Solid-State. Polymer 2016, 92, 58–65. [Google Scholar] [CrossRef]
- Montané, X.; Bhosale, S.V.; Reina, J.A.; Giamberini, M. Columnar Liquid Crystalline Polyglycidol De-rivatives: A Novel Alternative for Proton-Conducting Membranes. Polymer 2015, 66, 100–109. [Google Scholar] [CrossRef]
- Montané, X.; Bogdanowicz, K.A.; Colace, G.; Reina, J.A.; Cerruti, P.; Lederer, A.; Giamberini, M. Advances in the Design of Self-Supported Ion-Conducting Membranes-New Family of Columnar Liquid Crystalline Polyamines. Part 1: Copolymer Synthesis and Membrane Preparation. Polymer 2016, 105, 298–309. [Google Scholar] [CrossRef]
- Montané, X.; Bogdanowicz, K.A.; Prats-Reig, J.; Colace, G.; Reina, J.A.; Giamberini, M. Advances in the Design of Self-Supported Ion-Conducting Membranes—New Family of Columnar Liquid Crystalline Pol-yamines. Part 2: Ion Transport Characterisation and Comparison to Hybrid Membranes. Polymer 2016, 105, 234–242. [Google Scholar] [CrossRef]
- Ronda, J.C.; Reina, J.A.; Cádiz, V.; Giamberini, M.; Nicolais, L. Self-organized Liquid-crystalline Poly-ethers Obtained by Grafting Tapered Mesogenic Groups onto Poly (Epichlorohydrin): Toward Biomimetic Ion Channels. J. Polym. Sci. Part A Polym. Chem. 2003, 41, 2918–2929. [Google Scholar] [CrossRef]
- Šakalytė, A.; Reina, J.A.; Giamberini, M. Liquid Crystalline Polyamines Containing Side Dendrons: To-ward the Building of Ion Channels Based on Polyamines. Polymer 2013, 54, 5133–5140. [Google Scholar] [CrossRef]
- Percec, V.; Glodde, M.; Bera, T.K.; Miura, Y.; Shiyanovskaya, I.; Singer, K.D.; Balagurusamy, V.S.K.; Heiney, P.A.; Schnell, I.; Rapp, A. Self-Organization of Supramolecular Helical Dendrimers into Complex Electronic Materials. Nature 2002, 419, 384. [Google Scholar] [CrossRef] [PubMed]
- Gan, K.P.; Yoshio, M.; Kato, T. Columnar Liquid-Crystalline Assemblies of X-Shaped Pyrene–Oligothiophene Conjugates: Photoconductivities and Mechanochromic Functions. J. Mater. Chem. C 2016, 4, 5073–5080. [Google Scholar] [CrossRef] [Green Version]
- Soberats, B.; Yoshio, M.; Ichikawa, T.; Taguchi, S.; Ohno, H.; Kato, T. 3D Anhydrous Proton-Transporting Nanochannels Formed by Self-Assembly of Liquid Crystals Composed of a Sulfobetaine and a Sulfonic Acid. J. Am. Chem. Soc. 2013, 135, 15286–15289. [Google Scholar] [CrossRef]
- Badia, J.D.; Teruel-Juanes, R.; Acebo, C.; Gil-Castell, O.; Serra, A.; Ribes-Greus, A. Dielectric Spectroscopy of Novel Thiol-Ene/Epoxy Thermosets Obtained from Allyl-Modified Hyperbranched Poly(Ethyleneimine) and Diglycidylether of Bisphenol A. Eur. Polym. J. 2019, 113, 98–106. [Google Scholar] [CrossRef]
- Riande, E.; Díaz-Calleja, R. Electrical Properties of Polymers; CRC Press: London, UK, 2004. [Google Scholar]
- Salaberría, A.M.M.; Teruel-Juanes, R.; Badia, J.D.D.; Fernandes, S.C.C.M.; de Juano-Arbona, V.S.; Labidi, J.; Ribes-Greus, A. Influence of Chitin Nanocrystals on the Dielectric Behaviour and Conductivity of Chitosan-Based Bionanocomposites. Compos. Sci. Technol. 2018, 167, 323–330. [Google Scholar] [CrossRef]
- Torres-Giner, S.; Echegoyen, Y.; Teruel-Juanes, R.; Badia, J.; Ribes-Greus, A.; Lagaron, J. Electrospun Poly (Ethylene-Co-Vinyl Alcohol)/Graphene Nanoplatelets Composites of Interest in Intelligent Food Packaging Applications. Nanomaterials 2018, 8, 745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Šakalyte, A.; Reina, J.A.; Giamberini, M.; Lederer, A. Preparation of a Versatile Precursor of Novel Func-tionalized Polymers: The Influence of Polymerization Conditions on the Structure of Poly [1-(2-Hydroxyethyl)Aziridine]. Polym. Eng. Sci. 2014, 54, 579–591. [Google Scholar] [CrossRef]
- Lu, Z.; Manias, E.; Macdonald, D.D. Proceedings of the Electrochemical Society 204th National Meeting; The Pennsylvania State University: State College, PA, USA, 2003. [Google Scholar]
- Gross, G.W.; Johnson, J. The Layered Capacitor Method for Dielectric Bridge Measurements, Data Anal-ysis and Interpretation of Fluoride Doped Ice. IEEE Trans. Electr. Insul. 1983, EI-18, 485–497. [Google Scholar] [CrossRef]
- Teruel-Juanes, R. Propiedades Dieléctricas y Conductividad de Nuevos Electrolitos Poliméricos Para Aplicaciones Energéticas; Universitat Politècnica de València: Valencia, Spain, 2017. [Google Scholar]
- VonHippel, A.R. Dielectrics and Waves; Wiley: New York, NY, USA; Chapman & Hall: London, UK, 1954. [Google Scholar]
- Charlesworth, J.M. Deconvolution of Overlapping Relaxations in Dynamic Mechanical Spectra. J. Mater. Sci. 1993, 28, 399–404. [Google Scholar] [CrossRef]
- Havriliak, S.; Negami, S.A. A Complex Plane Analysis of alfa-dispersions in Some Polymer Systems. J. Polym. Sci. Part C Polym. Symp. 1966, 14, 99–117. [Google Scholar] [CrossRef]
- Havriliak, S.; Negami, S.A. A Complex Plane Representation of Dielectric and Mechanical Relaxation Processes in Some Polymers. Polymer 1967, 8, 161–210. [Google Scholar] [CrossRef]
- Fulcher, G.S. Analysis of Recent Measurements of the Viscosity of Glasses. J. Am. Ceram. Soc. 1992, 75, 1043–1055. [Google Scholar] [CrossRef]
- Vogel, H. The Temperature Dependence Law of the Viscosity of Fluids. Phys. Z 1921, 22, 645–646. [Google Scholar]
- Angell, C.A. Perspective on the glass transition. J. Phys. Chem. Solids 1988, 49, 863–871. [Google Scholar] [CrossRef]
- Angell, C.A. Relaxation in liquids, polymers and plastic crystals: Strong/fragile patterns and problems. J. Non-Crystalline Solids 1991, 131–133, 13–31. [Google Scholar] [CrossRef]
- Badia, J.D.; Monreal, L.; Juano-Arbona, V.S.D.; Ribes-Greus, A. Dielectric Spectroscopy of Recycled Polylactide. Polym. Degrad. Stab. 2014, 107, 21–27. [Google Scholar] [CrossRef] [Green Version]
- Badia, J.D.; Reig-Rodrigo, P.; Teruel-Juanes, R.; Kittikorn, T.; Strömberg, E.; Ek, M.; Karlsson, S.; Ribes-Greus, A. Effect of Sisal and Hydrothermal Ageing on the Dielectric Behaviour of Polylactide/Sisal Biocomposites. Compos. Sci. Technol. 2017, 149, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Felipe, A.; Santonja-Blasco, L.; Badia, J.D.; Imrie, C.T.; Ribes-Greus, A. Characterization of Functionalized Side-Chain Liquid Crystal Methacrylates Containing Nonmesogenic Units by Dielectric Spectroscopy. Ind. Eng. Chem. Res. 2013, 52, 8722–8731. [Google Scholar] [CrossRef]
- Maxwell, J.C. Electricity and Magnetism; Clarendon: Oxford, UK, 1892. [Google Scholar]
- Percec, V.; Heck, J.; Lee, M.; Ungar, G.; Alvarez-Castillo, A. Poly {2-Vinyloxyethyl 3, 4, 5-Tris [4-(n-Dodecanyloxy) Benzyloxy] Benzoate}: A Self-Assembled Supramolecular Polymer Similar to To-bacco Mosaic Virus. J. Mater. Chem. 1992, 2, 1033–1039. [Google Scholar] [CrossRef]
- Ronda, J.C.; Reina, J.A.; Giamberini, M. Self-organized Liquid-crystalline Polyethers Obtained by Grafting Tapered Mesogenic Groups onto Poly (Epichlorohydrin): Toward Biomimetic Ion Channels 2. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 326–340. [Google Scholar] [CrossRef]
- Qin, Q.; McKenna, G.B. Correlation between Dynamic Fragility and Glass Transition Temperature for Different Classes of Glass Forming Liquids. J. Non. Cryst. Solids 2006, 352, 2977–2985. [Google Scholar] [CrossRef]
- Adam, G.; Gibbs, J.H. On the Temperature Dependence of Cooperative Relaxation Properties in Glass-forming Liquids. J. Chem. Phys. 1965, 43, 139–146. [Google Scholar] [CrossRef] [Green Version]
- Turnbull, D.; Cohen, M.H. Free-volume Model of the Amorphous Phase: Glass Transition. J. Chem. Phys. 1961, 34, 120–125. [Google Scholar] [CrossRef]















| Δ ε | PAZE40 | Δ ε | PAZE100 | ||||||
| T (K) | 153 | 163 | 173 | T (K) | 153 | 163 | 173 | ||
| γ | 0.001 | 0.001 | 0.002 | γ | 0.001 | 0.001 | 0.001 | ||
| γ-O | 0.005 | 0.003 | 0.002 | γ-O | 0.016 | 0.019 | 0.018 | ||
| T (K) | 253 | 263 | 273 | T (K) | 293 | 303 | 313 | ||
| αTg | 0.049 | 0.051 | 0.087 | αTg | 0.003 | 0.004 | 0.006 | ||
| αTg-O | 0.017 | 0.022 | 0.041 | αTg-O | 0.007 | 0.007 | 0.020 | ||
| T (K) | 293 | 303 | 313 | T (K) | 313 | 323 | 333 | ||
| αClear | 0.080 | 0.176 | 0.211 | αClear | 0.242 | 0.252 | 0.312 | ||
| αClear-O | 0.083 | 0.083 | 0.098 | αClear-O | 1.197 | 1.409 | 1.926 | ||
| Material | logf0 (Hz) | Ea (kJ·mol−1) | Tmax 1 kHz (K) | R2 |
|---|---|---|---|---|
| PAZE100 | 12.5 | 23.5 | 128.6 | 0.99 |
| PAZE100-O | 30.9 | 78.9 | 147.5 | 0.99 |
| PAZE40 | 13.1 | 24.6 | 127.6 | 0.99 |
| PAZE40-O | 15.4 | 33.9 | 143.5 | 0.97 |
| αTg | τ0 (s) | D | TVFTH (K) | ФTg | αTg × 104 (K−1) | R2 |
|---|---|---|---|---|---|---|
| PAZE100 | 11.8 ± 0.4 | 8.5±1.4 | 180 ± 6 | 0.033 | 6.5 | 0.99 |
| PAZE100-O | 8.6 ± 0.7 | 3.9 ± 0.5 | 180 ± 6 | 0.072 | 14.3 | 0.94 |
| PAZE40 | 8.1 ± 0.4 | 5.0 ± 0.8 | 183 ± 4 | 0.055 | 11.0 | 0.99 |
| PAZE40-O | 6.1 ± 0.5 | 5.7 ± 0.5 | 184 ± 4 | 0.048 | 9.5 | 0.94 |
| αClear | τ0 (s) | D | TVFTH (K) | ФTg | αTg × 104 (K−1) | R2 |
|---|---|---|---|---|---|---|
| PAZE100 | 4.9 ± 0.8 | 1.4 ± 0.2 | 278 | --- | --- | 0.92 |
| PAZE100-O | 7.1 ± 0.2 | 2.1 ± 0.1 | 278 | 0.086 | 17.2 | 0.99 |
| PAZE40 | 7.2 ± 0.5 | 2.5 ± 0.2 | 268 | 0.075 | 15.1 | 0.98 |
| PAZE40-O | 7.6 ± 1.0 | 2.5 ± 0.4 | 268 | 0.075 | 15.0 | 0.93 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teruel-Juanes, R.; Bogdanowicz, K.A.; Badia, J.D.; Sáenz de Juano-Arbona, V.; Graf, R.; Reina, J.A.; Giamberini, M.; Ribes-Greus, A. Molecular Mobility in Oriented and Unoriented Membranes Based on Poly[2-(Aziridin-1-yl)ethanol]. Polymers 2021, 13, 1060. https://doi.org/10.3390/polym13071060
Teruel-Juanes R, Bogdanowicz KA, Badia JD, Sáenz de Juano-Arbona V, Graf R, Reina JA, Giamberini M, Ribes-Greus A. Molecular Mobility in Oriented and Unoriented Membranes Based on Poly[2-(Aziridin-1-yl)ethanol]. Polymers. 2021; 13(7):1060. https://doi.org/10.3390/polym13071060
Chicago/Turabian StyleTeruel-Juanes, Roberto, Krzysztof Artur Bogdanowicz, Jose D. Badia, Victor Sáenz de Juano-Arbona, Robert Graf, Jose A. Reina, Marta Giamberini, and Amparo Ribes-Greus. 2021. "Molecular Mobility in Oriented and Unoriented Membranes Based on Poly[2-(Aziridin-1-yl)ethanol]" Polymers 13, no. 7: 1060. https://doi.org/10.3390/polym13071060
APA StyleTeruel-Juanes, R., Bogdanowicz, K. A., Badia, J. D., Sáenz de Juano-Arbona, V., Graf, R., Reina, J. A., Giamberini, M., & Ribes-Greus, A. (2021). Molecular Mobility in Oriented and Unoriented Membranes Based on Poly[2-(Aziridin-1-yl)ethanol]. Polymers, 13(7), 1060. https://doi.org/10.3390/polym13071060