Preparation and Characterization of New Sol–Gel Hybrid Inulin–TEOS Adsorbent
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Synthesis of Inulin–TEOS Adsorbent
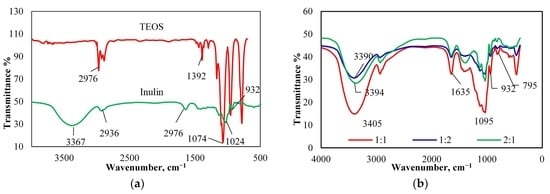
3.2. Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sarode, S.; Upadhyay, P.; Khosa, M.A.; Mak, T.; Shakir, A.; Song, S.; Ullah, A. Overview of wastewater treatment methods with special focus on biopolymer chitin-chitosan. Int. J. Biol. Macromol. 2019, 121, 1086–1100. [Google Scholar] [CrossRef]
- Vakili, M.; Rafatullah, M.; Salamatinia, B.; Abdullah, A.Z.; Ibrahim, M.H.; Tan, K.B.; Gholami, Z.; Amouzgar, P. Application of chitosan and its derivatives as adsorbents for dye removal from water and wastewater: A review. Carbohydr. Polym. 2014, 113, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Giri, A.; Bhowmick, R.; Prodhan, C.; Majumder, D.; Bhattacharya, S.K.; Ali, M. Synthesis and characterization of biopolymer-based hybrid hydrogel nanocomposite and study of their electrochemical efficacy. Int. J. Biol. Macromol. 2018, 123, 228–238. [Google Scholar] [CrossRef]
- Prasanna, S.B.; Selvakumar, D.; Kadirvelu, K.; Kumar, N.S. Chitosan as an environment friendly biomaterial—A review on recent modifications and applications. Int. J. Biol. Macromol. 2020, 150, 1072–1083. [Google Scholar]
- Argüello, L.; Hernandez-Martínez, A.R.; Rodríguez, A.; Molina, G.A.; Esparza, R.; Estevez, M. Novel chitosan/polyurethane/anatase titania porous hybrid composite for removal of metal ions waste. J. Chem. Technol. Biotechnol. 2016, 9, 2185–2197. [Google Scholar] [CrossRef]
- Hernández-Martínez, A.R.; Molina, G.A.; Jiménez-Hernández, L.F.; Oskam, A.H.; Fonseca, G.; Estevez, M. Evaluation of Inulin Replacing Chitosan in a Polyurethane/Polysaccharide Material for Pb2+ Removal. Molecules 2017, 22, 2093. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, R.; Mirza, A. Adsorption of Pb(II) and Cu(II) by Alginate-Au-Mica bionanocompositeKinetic, isotherm and thermodynamic studies. Process. Saf. Environ. 2017, 109, 1–10. [Google Scholar] [CrossRef]
- Yu, K.; Ho, J.; McCandlish, E.; Buckley, B.; Patel, R.; Li, Z.; Shapley, N.C. Copper ion adsorption by chitosan nanoparticles and alginate microparticles for water purification applications. Colloids Surf. A Phys. Eng. 2013, 425, 31–41. [Google Scholar] [CrossRef]
- Rio, S.; Martin, P. Removal of metal ions from aqueous solution by adsorption onto low-cost biosorbent. Environ. Technol. 2012, 33, 2211–2215. [Google Scholar] [CrossRef]
- Li, K.; Liu, S.; Xing, R.; Yu, H.; Qin, Y.; Li, P. Liquid phase adsorption behavior of inulin-type fructan onto activated charcoal. Carbohydr. Polym. 2015, 122, 237–242. [Google Scholar] [CrossRef]
- Sahiner, N.; Sagbas, S. Multifunctional tunable p(inulin) microgels. Mater. Sci. Eng. C 2014, 40, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Danks, A.E.; Hall, S.R.; Schnepp, Z. The evolution of “sol-gel” chemistry as a technique for materials synthesis. Mater. Horiz. 2016, 3, 91–112. [Google Scholar] [CrossRef] [Green Version]
- Amiri, S.; Rahimi, A. Hybrid nanocomposite coating by sol–gel method: A review. Iran. Polym. J. 2016, 25, 559–577. [Google Scholar] [CrossRef]
- Budnyak, T.M.; Aminzadeh, S.; Pylypchuk, L.V.; Sternik, D.; Tertykh, V.A.; Lindstrom, M.E.; Sevastyanova, O. Methylene Blue dye sorption by hybrid materials from technical lignins. J. Environ. Chem. Eng. 2018, 6, 4997–5007. [Google Scholar] [CrossRef]
- Carrera-Figueiras, C.; Pérez-Padilla, Y.; Estrella-Gutiérrez, M.A.; Uc-Cayetano, E.G.; Juárez-Moreno, J.A.; Avila-Ortega, A. Surface Science Engineering through Sol-Gel Process. Appl. Surf. Sci. Gurrappa InjetiIntechopen 2019. [Google Scholar] [CrossRef] [Green Version]
- Esposito, S. “Traditional” sol-gel chemistry as a powerful tool for the preparation of supported metal and metal oxide catalysts. Materials 2019, 12, 668. [Google Scholar] [CrossRef] [Green Version]
- Qu, H.; Bhattacharyya, S.; Ducheyne, P. Sol-gel processed oxide controlled release materials. In Comprehensive Biomaterials II, 2nd ed.; Ducheyne, P., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 4, pp. 617–643. [Google Scholar] [CrossRef]
- Padmaja, G.V. Preparation of Orthosilicic Acid by Sol-Gel Technique using Tetraethyl orthosilicic acid (TEOS) and its applications. Int. J. Sci. Sci. Res. 2015, 5, 2250–3153. [Google Scholar]
- Wan Ibrahim, W.A.; Wan Ismail, W.N.; Abdul Keyon, A.S.; Sanag, M.M. Preparation and Characterization of a New Sol–gel Hybrid Based Tetraethoxysilane-polydimethylsiloxane as a Stir Bar Extraction Sorbent Materials. J. Sol. Gel. Sci. Sci. Technol. 2011, 58, 602–661. [Google Scholar] [CrossRef] [Green Version]
- Kołodyńska, D.; Budnyak, T.M.; Hubicki, Z.; Tertykh, V.A. Sol–Gel Derived Organic–Inorganic Hybrid Ceramic Materials for Heavy Metal Removal. In Sol-Gel Based Nanoceramic Materials: Preparation, Properties and Applications, 1st ed.; Mishra, A.K., Ed.; Springer: Cham, Switzerland; Johannesburg, South Africa, 2016; pp. 253–274. [Google Scholar] [CrossRef]
- Barberena-Fernández, A.M.; Carmona-Quiroga, P.M.; Blanco-Varela, M.T. Interaction of TEOS with cementitious materials: Chemical and physical effects. Cem. Concr. Compos. 2015, 55, 145–152. [Google Scholar] [CrossRef] [Green Version]
- Al-Sagheer, F.; Muslim, S. Thermal and mechanical properties of chitosan/SiO2 hybrid composites. J. Nanomater. 2010, 1–2, 3. [Google Scholar]
- Prerna, D.; Ramanand, J. Comparative study of physical and thermal properties of Chitosan Silica hybrid coatings prepared by sol-gel method. Pelagia Reseach Libr. Der Chem. Sin. 2012, 3, 589–601. [Google Scholar]
- Yeh, J.T.; Chen, C.L.; Huang, K.S. Synthesis and Properties of Chitosan/SiO2 Hybrid Materials. Mater. Lett. 2007, 61, 1292–1295. [Google Scholar] [CrossRef]
- Wen, L.; Manlin, L.; Shuncheng, J.; Amjad, A.; Zengqiang, Z.; Ronghua, L. Polyamine-co-2, 6-diaminopyridine covalently bonded on chitosan for the adsorptive removal of Hg(II) ions from aqueous solution. Int. J. Biol. Macromol. 2019, 130, 853–862. [Google Scholar]
- Hajiaghababaei, L.; Badiei, A.; Ganjali, M.R.; Heydari, S.; Khaniani, Y.; Ziarani, G.M. Highly efficient removal and preconcentration of lead and cadmium cations from water and wastewater samples using ethylenediamine functionalized SBA-15. Desalination 2011, 266, 182–187. [Google Scholar] [CrossRef]
- Tana, P.; Jansawang, N.; Pimchan, P. The Preparation of Hybrid Material of Cobalt Complex into Mesoporous Silica from the Rice Husk. Suan Sunandha Sci. Technol. J. 2020, 7, 7–14. [Google Scholar]
- Prashanth, S.K.; Leon, K.; Keesman, K.J.; Loosdrecht, M.C.M.; Witkamp, G. Effect of pore size distribution and particle size of porous metal oxides on phosphate adsorption capacity and kinetics. Chem. Eng. J. 2019, 358, 160–169. [Google Scholar]
- Bello, O.S.; Adegoke, K.A.; Akinyunni, O.O. Preparation and characterization of a novel adsorbent from Moringa oleifera leaf. Appl. Water Sci. 2017, 7, 1295–1305. [Google Scholar] [CrossRef] [Green Version]
- Todorova, E.V.; Chernev, G.E.; Djambazov, S.P. Structure and properties of functionalized porous silica hybrid materials. Open J. Inorg. Non-Metal. Mater. 2014, 4, 35–43. [Google Scholar] [CrossRef] [Green Version]
- Kow, K.H.; Yusoff, R.; Abdul Aziz, A.R.; Abdullah, E.C. From bamboo leaf to aerogel: Preparation of water glass as a precursor. J. Non-Cryst. Solids 2014, 386, 76–84. [Google Scholar] [CrossRef]
- Ronkart, S.N.; Paquot, M.; Fougnies, C.; Deroanne, C.; Blecker, C.S. Effect of water uptake on amorphous inulin properties. Food Hydrocoll. 2009, 23, 922–927. [Google Scholar] [CrossRef]
- Shahkarami, S.; Dalai, A.; Soltan, J. Enhanced CO 2 Adsorption Using MgO-Impregnated Activated Carbon: Impact of Preparation Techniques. Ind. Eng. Chem. Res. 2016, 55, 5955–5964. [Google Scholar] [CrossRef]
- Gutiérrez-Castoren, M.C.; Effland, W.R. Pedogenic and Biogenic Siliceous Features. In Interpretation of Micromorphological Features of Soils and Regoliths, 2nd ed.; Georges, S., Vera, M., Florias, M., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2010; pp. 471–496. [Google Scholar]
- Skwarek, E.; Goncharuk, O.; Sternik, D. Synthesis, Structural, and Adsorption Properties and Thermal Stability of Nanohydroxyapatite/Polysaccharide Composites. Nanoscale Res. Lett. 2017, 12, 155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, G.S.; Oliveira, P.C.; Giordani, D.S.; De Castro, H.F. Chitosan/siloxane hybrid polymer: Synthesis, characterization and performance as a support for immobilizing enzyme. J. Braz. Chem. Soc. 2011, 22, 1407–1417. [Google Scholar] [CrossRef]
- Linlin, Z.; Ru, L.; Tianwei, D.; Lu, W.; Jun, M.; Liguo, S. Electrospun superhydrophilic membranes for effective removal of Pb(II) from water. Nanoscale Adv. 2019, 1, 389–394. [Google Scholar]










| Element | Weight % | Atomic % |
|---|---|---|
| Carbon | 40.122 | 48.490 |
| Oxygen | 51.818 | 47.016 |
| Silicon | 5.193 | 2.684 |
| Sodium | 2.867 | 1.810 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohd Yusop, H.; Mohd Ismail, A.I.H.; Wan Ismail, W.N. Preparation and Characterization of New Sol–Gel Hybrid Inulin–TEOS Adsorbent. Polymers 2021, 13, 1295. https://doi.org/10.3390/polym13081295
Mohd Yusop H, Mohd Ismail AIH, Wan Ismail WN. Preparation and Characterization of New Sol–Gel Hybrid Inulin–TEOS Adsorbent. Polymers. 2021; 13(8):1295. https://doi.org/10.3390/polym13081295
Chicago/Turabian StyleMohd Yusop, Hartina, Annur Isma Husna Mohd Ismail, and Wan Norfazilah Wan Ismail. 2021. "Preparation and Characterization of New Sol–Gel Hybrid Inulin–TEOS Adsorbent" Polymers 13, no. 8: 1295. https://doi.org/10.3390/polym13081295
APA StyleMohd Yusop, H., Mohd Ismail, A. I. H., & Wan Ismail, W. N. (2021). Preparation and Characterization of New Sol–Gel Hybrid Inulin–TEOS Adsorbent. Polymers, 13(8), 1295. https://doi.org/10.3390/polym13081295