Hydroxyl-Terminated Saponified Natural Rubber Based on the H2O2/P25-TiO2 Powder/UVC-Irradiation System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
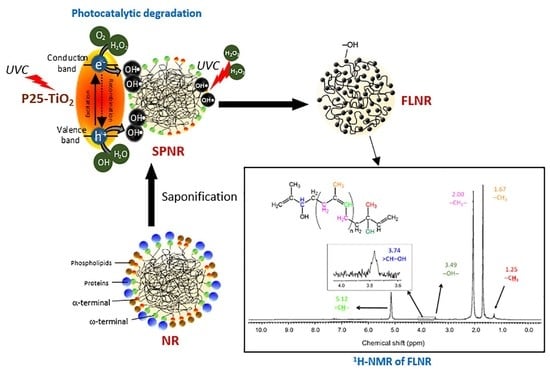
2.2.1. Preparation of SPNR
2.2.2. Preparation of FLNR
2.3. Characterizations
2.3.1. Determination of the Crystallinity of TiO2 Powder
2.3.2. Determination of the Chemical Structure of the Rubber Samples
3. Results and Discussion
3.1. Characterization of TiO2 Powder
3.2. Effect of the TiO2 Powder Content on the MW and the Structure of SPNR and FLNR
3.3. Effect of H2O2 Content on the MW and the Structure of SPNR and FLNR
3.4. Effect of UVC-Irradiation Time on the SPNR and FLNR Structures
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tarachiwin, L.; Sakdapipanich, J.; Ute, K.; Kitayama, T.; Tanaka, Y. Structural Characterization of α-Terminal Group of Natural Rubber. 2. Decomposition of Branch-Points by Phospholipase and Chemical Treatments. Biomacromolecules 2005, 6, 1858–1863. [Google Scholar] [CrossRef]
- Lake, G.J.; Samsuri, A.; Teo, S.C.; Vaja, J. Time-Dependent Fracture in Vulcanized Elastomers. Polymer 1991, 32, 2963–2975. [Google Scholar] [CrossRef]
- Srilathakutty, R.; John, N.; Joseph, R.; George, K.E. Use of Amine Terminated Liquid Natural Rubber as a Plasticiser in Filled NR and NBR Compounds. Int. J. Polym. Mater. Polym. Biomater. 1996, 32, 235–246. [Google Scholar] [CrossRef]
- Mounir, A.; Darwish, N.A.; Shehata, A. Effect of Maleic Anhydride and Liquid Natural Rubber as Compatibilizers on the Mechanical Properties and Impact Resistance of the NR-NBR Blend. Polym. Adv. Technol. 2004, 15, 209–213. [Google Scholar] [CrossRef]
- Dahlan, H.M.; Zaman, M.D.K.; Ibrahim, A. Liquid Natural Rubber (LNR) as a Compatibiliser in NR/LLDPE Blends–II: The Ef-fects of Electron-beam (EB) Irradiation. Radiat. Phys. Chem. 2002, 64, 429–436. [Google Scholar] [CrossRef]
- Phetphaisit, C.W.; Bumee, R.; Namahoot, J.; Ruamcharoen, J.; Ruamcharoen, P. Polyurethane Polyester Elastomer: Innovative Environmental Friendly Wood Adhesive from Modified PETs and Hydroxyl Liquid Natural Rubber Polyols. Int. J. Adhes. Adhes. 2013, 41, 127–131. [Google Scholar] [CrossRef]
- Kaenhin, L.; Klinpituksa, P.; Rungvichaniwat, A.; Pilard, J.-F. Waterborne Polyurethane: Effect of Functional Groups in Aromatic Isocyanate and the Chain Length of Hydroxyl Terminated Natural Rubber. Adv. Mater. Res. 2012, 415–417, 2032–2035. [Google Scholar] [CrossRef]
- Saetung, A.; Kaenhin, L.; Klinpituksa, P.; Rungvichaniwat, A.; Tulyapitak, T.; Munleh, S.; Campistron, I.; Pilard, J.-F. Synthesis, Characteristic, and Properties of Waterborne Polyurethane Based on Natural Rubber. J. Appl. Polym. Sci. 2012, 124, 2742–2752. [Google Scholar] [CrossRef]
- Kébir, N.; Campistron, I.; Laguerre, A.; Pilard, J.-F.; Bunel, C. New Crosslinked Polyurethane Elastomers with Various Physical Properties from Natural Rubber Derivatives. J. Appl. Polym. Sci. 2011, 122, 1677–1687. [Google Scholar] [CrossRef]
- Ly, P.H. Reinforcement of Natural Rubber from Hydroxyl-Terminated Liquid Natural Rubber Grafted Carbon Black. I. Grafting of Acyl Chloride Capped Liquid Natural Rubber onto Carbon Black. J. Macromol. Sci. Part A Pure Appl. Chem. 1996, 33, 1931–1937. [Google Scholar] [CrossRef]
- Isa, S.Z.; Yahya, R.; Hassan, A.; Tahir, M. The Influence of Temperature and Reaction Time in the Degradation of Natural Rubber Latex. Malays. J. Anal. Sci. 2007, 11, 42–47. [Google Scholar]
- Ibrahim, S.; Daik, R.; Abdullah, I. Functionalization of Liquid Natural Rubber via Oxidative Degradation of Natural Rubber. Polymers 2014, 6, 2928–2941. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, S.; Sreekantan, S.; Tan, K.S.; Nor, Z.M.; Ismail, H. Preparation and Characterization of Low-Molecular-Weight Natural Rubber Latex via Photodegradation Catalyzed by Nano TiO2. Polymers 2018, 10, 1216. [Google Scholar] [CrossRef] [Green Version]
- Ravindran, T.; Nayar, M.R.G.; Francis, D.J. Production of Hydroxyl-Terminated Liquid Natural Rubber—Mechanism of Photochemical Depolymerization and Hydroxylation. J. Appl. Polym. Sci. 1988, 35, 1227–1239. [Google Scholar] [CrossRef]
- Nijpanich, S.; Nimpaiboon, A.; Sakdapipanich, J. Functionalization of Styrene-Butadiene Rubber and Skim Latex by Photo-Catalytic Reaction Using Nanometric TiO2 Film as a Photocatalyst. Key Eng. Mater. 2015, 659, 409–413. [Google Scholar] [CrossRef]
- Negishi, N.; Takeuchi, K.; Ibusuki, T. The Surface Structure of Titanium Dioxide Thin Film Photocatalyst. Appl. Surf. Sci. 1997, 121–122, 417–420. [Google Scholar] [CrossRef]
- Nawamawat, K.; Sakdapipanich, J.T.; Ho, C.C.; Ma, Y.; Song, J.; Vancso, J.G. Surface Nanostructure of Hevea brasiliensis Natural Rubber Latex Particles. Colloids Surf. A 2011, 390, 157–166. [Google Scholar] [CrossRef]
- Yunyongwattanakorn, J.; Tanaka, Y.; Sakdapipanich, J.; Wongsasutthikul, V. Highly-Purified Natural Rubber by Saponification of Latex: Analysis of Residual Proteins in Saponified Natural Rubber. Rubber Chem. Technol. 2008, 81, 121–137. [Google Scholar] [CrossRef]
- Hurum, D.C.; Agrios, A.G.; Gray, K.A.; Rajh, T.; Thurnauer, M.C. Explaining the Enhanced Photocatalytic Activity of Degussa P25 Mixed-Phase TiO2 Using EPR. J. Phys. Chem. B 2003, 107, 4545–4549. [Google Scholar] [CrossRef]
- Dafader, N.C.; Haque, M.E.; Akhtar, F.; Ahmad, M.U. Study on Grafting of Different Types of Acrylic Monomers onto Natural Rubber by γ-rays. Radiat. Phys. Chem. 2006, 75, 168–172. [Google Scholar] [CrossRef]
- Alaton, I.A.; Balcioglu, I.A.; Bahnemann, D.W. Advanced Oxidation of a Reactive Dyebath Effluent: Comparison of O3, H2O2/UV-C and TiO2/UV-A Processes. Water Res. 2002, 36, 1143–1154. [Google Scholar] [CrossRef]
- Phinyocheep, P.; Phetphaisit, C.W.; Derouet, D.; Campistron, I.; Brosse, J.C. Chemical Degradation of Epoxidized Natural Rubber Using Periodic acid: Preparation of Epoxidized Liquid Natural Rubber. J. Appl. Polym. Sci. 2005, 95, 6–15. [Google Scholar] [CrossRef]
- Sakdapipanich, I.; Nawamawat, K. Effect of treatment of skim natural rubber latex on properties of pressure-sensitive adhesive tapes. Kauchuk i Rezina 2005, 5, 9–11. [Google Scholar]
- Saetung, A.; Rungvichaniwat, A.; Campistron, I.; Klinpituksa, P.; Laguerre, A.; Phinyocheep, P.; Doutres, O.; Pilard, J.-F. Preparation and Physico-Mechanical, Thermal and Acoustic Properties of Flexible Polyurethane Foams Based on Hydroxytelechelic Natural Rubber. J. Appl. Polym. Sci. 2010, 117, 828–837. [Google Scholar] [CrossRef]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nijpanich, S.; Nimpaiboon, A.; Rojruthai, P.; Sakdapipanich, J. Hydroxyl-Terminated Saponified Natural Rubber Based on the H2O2/P25-TiO2 Powder/UVC-Irradiation System. Polymers 2021, 13, 1319. https://doi.org/10.3390/polym13081319
Nijpanich S, Nimpaiboon A, Rojruthai P, Sakdapipanich J. Hydroxyl-Terminated Saponified Natural Rubber Based on the H2O2/P25-TiO2 Powder/UVC-Irradiation System. Polymers. 2021; 13(8):1319. https://doi.org/10.3390/polym13081319
Chicago/Turabian StyleNijpanich, Supinya, Adun Nimpaiboon, Porntip Rojruthai, and Jitladda Sakdapipanich. 2021. "Hydroxyl-Terminated Saponified Natural Rubber Based on the H2O2/P25-TiO2 Powder/UVC-Irradiation System" Polymers 13, no. 8: 1319. https://doi.org/10.3390/polym13081319
APA StyleNijpanich, S., Nimpaiboon, A., Rojruthai, P., & Sakdapipanich, J. (2021). Hydroxyl-Terminated Saponified Natural Rubber Based on the H2O2/P25-TiO2 Powder/UVC-Irradiation System. Polymers, 13(8), 1319. https://doi.org/10.3390/polym13081319