Fibrous Polymer-Based Composites Obtained by Electrospinning for Bone Tissue Engineering
Abstract
:1. Introduction
2. Modern Polymer-Based Fibrous Composites
2.1. Polymers for ECM Imitation in BTE
2.2. Polymer Materials Incorporated with Inorganic Components
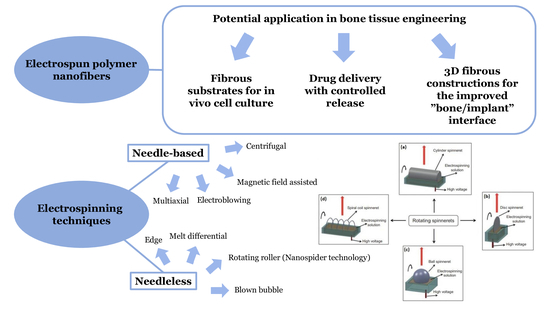
3. Application of Electrospinning Techniques for Fibrous Scaffold Fabrication
3.1. Electrospinning Principle
3.2. Advantages and Key Issues of Electrospinning
4. Conclusions
5. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dhandayuthapani, B.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Polymeric Scaffolds in Tissue Engineering Application: A Review. Int. J. Polym. Sci. 2011, 2011, 290602. [Google Scholar] [CrossRef]
- Lei, T.; Zhang, T.; Ju, W.; Chen, X.; Heng, B.C.; Shen, W.; Yin, Z. Biomimetic strategies for tendon/lingament-to-bone interface regeneration. Bioact. Mater. 2021, 6, 2491–2510. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, L.; Fan, Y.; Feng, Q.; Cui, F.-Z.; Watari, F. Nanostructured scaffolds for bone tissue engineering. J. Biomed. Mater. Res. Part A 2013, 101, 2424–2435. [Google Scholar] [CrossRef] [PubMed]
- Nosoudi, N.; Karamched, K.; Holman, D.; Lei, Y.; Rodriguez-Devora, J. Engineered extracellular matrix: Current accoplishments and future trends. J. Biomed. Mater. Res. A 2014, 1, 1–15. [Google Scholar]
- Molnar, K.; Nagy, Z.K. Corona-electrospinning: Needleless method for high-throughput continuous nanofiber production. Eur. Polym. J. 2016, 74, 279–286. [Google Scholar] [CrossRef]
- Alyah, K.; Cartmell, S.H.; Richardson, S.M.; Bosworth, L.A. Material characterization of PCL:PLLA electrospun fibers following six month degradation in vitro. Polymers 2020, 12, 700–711. [Google Scholar]
- Seddighian, A.; Ganji, F.; Baghaban-Eslaminejad, M.; Bagheri, F. Electrospun PCL scaffold modified with chitosan nanoparticles for enhanced bone regeneration. Prog. Biomater. 2021, 10, 65–76. [Google Scholar] [CrossRef]
- Grabska-Zielińska, S.; Sionkowska, A.; Carvalho, Â.; Monteiro, F. Biomaterials with Potential Use in Bone Tissue Regeneration—Collagen/Chitosan/Silk Fibroin Scaffolds Cross-Linked by EDC/NHS. Materials 2021, 14, 1105. [Google Scholar] [CrossRef]
- Aldana, A.A.; Abraham, G.A. Current advances in electrospun gelatin-based scaffolds for tissue engineering applications. Int. J. Pharm. 2017, 25, 441–453. [Google Scholar] [CrossRef] [Green Version]
- Chen, R.; Qiu, L.; Ke, Q.; He, C.; Mo, X. Electrospinning Thermoplastic Polyurethane-Contained Collagen Nanofibers for Tissue-Engineering Applications. J. Biomater. Sci. Polym. Ed. 2009, 20, 1513–1536. [Google Scholar] [CrossRef]
- Hild, M.; Toskas, G.; Aibibu, D.; Wittenburg, G.; Meissner, H.; Cherif, C.; Hund, R.-D. Chitosan/gelatin micro/nanofiber 3D composite scaffolds for regenerative medicine. Compos. Interfaces 2014, 21, 301–308. [Google Scholar] [CrossRef]
- Keshvardoostchokami, M.; Majidi, S.S.; Huo, P.; Ramachandran, R.; Chen, M.; Liu, B. Electrospun nanofibers of natural and synthetic polymers as artificial extracellular matrix for tissue enginnering. Nanomaterials 2021, 11, 21. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, Y.; Yan, J.; Zhang, K.; Lin, F.; Xiang, L.; Deng, L.; Guan, Z.; Cui, W.; Zhang, H. Pharmaceutical electrospinning and 3D printing scaffold design for bone regeneration. Adv. Drug Deliv. Rev. 2021, 174, 504–534. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Patil, S.; Gao, Y.-G.; Qian, A. The Bone Extracellular Matrix in Bone Formation and Regeneration. Front. Pharmacol. 2020, 11, 757–772. [Google Scholar] [CrossRef]
- Samadian, H.; Khastar, H.; Ehterami, A.; Salehi, M. Bioengineered 3D nanocomposite based on gold nanoparticles and gelatin nanofibers for bone regeneration: In vitro and in vivo study. Nature 2021, 11, 13877–13888. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, J. Electrospun poly(lactic-co-glycolic acid)/wool keratin fibrous composite scaffolds potential for bone tissue engineering applications. J. Bioact. Conpat. Polym. 2013, 28, 141–153. [Google Scholar] [CrossRef]
- Wu, J.; Cao, L.; Liu, Y.; Zheng, A.; Jiao, D.; Zeng, D.; Wang, X.; Kaplan, D.L.; Jiang, X. Functionalization of Silk Fibroin Electrospun Scaffolds via BMSC Affinity Peptide Grafting through Oxidative Self-Polymerization of Dopamine for Bone Regeneration. ACS Appl. Mater. Interfaces 2019, 11, 8878–8895. [Google Scholar] [CrossRef]
- Sreerekha, P.R.; Chennazhi, K.P.; Nair, S.V.; Menon, D.; Afeesh, R. A novel method for the fabrication of fibrin-based electrospun nanofibrous scaffold for tissue-engineering applications. Tissue Eng. Part C Methods 2011, 17, 1121–1130. [Google Scholar]
- Ko, E.; Lee, J.S.; Kim, H.; Yang, S.Y.; Yang, D.; Yang, K.; Lee, J.; Shin, J.; Yang, H.S.; Ryu, W.; et al. Electrospun silk fibroin nanofibrous scaffolds with two-stage hydroxyapatite fubctionalization for enhancing the osteogenic differentiation of human adipose-derived mesenchymal stem cells. ACS Appl Mater Interfaces 2017, 10, 7614–7625. [Google Scholar] [CrossRef]
- Wang, F.; Hu, S.; Jia, Q.; Zhang, L. Advances in Electrospinning of Natural Biomaterials for Wound Dressing. J. Nanomater. 2020, 2020, 8719859. [Google Scholar] [CrossRef] [Green Version]
- Patil, T.V.; Patel, D.K.; Dutta, S.D.; Ganguly, K.; Santra, T.S.; Lim, K.T. Bioactive electrospun nanocomposite scaffolds of poly(lactic acid)/cellulose nanocrystals for bone tissue en-gineering. Int. J. Biol. Macromol. 2020, 162, 1429–1441. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.I.; Krebs, M.D.; Bonino, C.A.; Khan, S.A.; Alsberg, E. Electrospun alginate nanofibers with controlled cell adhesion for tissue engineering. Macromol. Biosci. 2010, 10, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Liu, X.; Zhou, X.; Zhang, H.; Zhang, W.; Xiao, W.; Pan, G.; Cui, W.; Santos, H.A.; Shi, Q. Gelatin Templated Polypeptide Co-Cross-Linked Hydrogel for Bone Regeneration. Adv. Health Mater. 2019, 9, e1901239. [Google Scholar] [CrossRef] [PubMed]
- Noh, Y.K.; Dos Santos Da Costa, A.; Park, Y.S.; Du, P.; Kim, I.H.; Park, K. Fabrication of bacterial cellulose-collagen composite scaffolds and their osteogenic effect on human mesen-chymal stem cells. Carbohydr. Polym. 2019, 219, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Yeo, M.; Ahn, S.; Kang, D.O.; Jang, C.H.; Lee, H.; Park, G.-M.; Kim, G.H. Designed hybrid scaffolds consisting of polycaprolactone microstrands and electrospun collagen-nanofibers for bone tissue regeneration. J. Biomed. Mater. Res. B Appl. Biomater. 2011, 97, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, Y.; He, W.; Huang, Q.; Zhang, R.; Feng, Q. Hydroxyapatite/collagen coating on PLGA electrospun fibers for osteogenic differentiation of bone marrow mesenchymal stem cells. J. Biomed. Mater. Res. Part A 2018, 106, 2863–2870. [Google Scholar] [CrossRef]
- Lai, G.-J.; Shalumon, K.T.; Chen, S.H.; Chen, J.P. Composite chitosan/silk fibroin nanofibers for modulation of osteogenic differentiation and proliferation of human mesenchymal stem cells. Carbohydr. Polym. 2014, 111, 288–297. [Google Scholar] [CrossRef]
- Bhattacharjee, P.; Kundu, B.; Naskar, D.; Maiti, T.K.; Bhattacharya, D.; Kundu, S.C. Nanofibrous nonmulberry silk/PVA scaffold for osteoinduction and osseointegration. Biopolymers 2015, 103, 271–284. [Google Scholar] [CrossRef]
- Noorani, B.; Tabandeh, F.; Yazdian, F.; Soheili, Z.-S.; Shakibaie, M.; Rahmani, S. Thin natural gelatin/chitosan nanofibrous scaffolds for retinal pigment epithelium cells. Int. J. Polym. Mater. 2018, 67, 754–763. [Google Scholar] [CrossRef]
- Righi, T.M.; Almeida, R.S.; D’ávila, M.A. Electrospinning of Gelatin/PEO Blends: Influence of Process Parameters in the Nanofiber Properties. Macromol. Symp. 2012, 319, 230–234. [Google Scholar] [CrossRef]
- Anjum, F.; Agabalyan, N.A.; Sparks, H.; Rosin, N.L.; Kallos, M.S.; Biernaskie, J. Biocomposite nanofiber matrices to support ECM remodeling by human dermal progenitors and enhanced wound closure. Sci. Rep. 2017, 7, 10291. [Google Scholar] [CrossRef]
- Teng, S.-H.; Wang, P.; Kim, H.E. Blend fibers of chitosan-agarose by electrospinning. Mater. Lett. 2009, 63, 2510–2512. [Google Scholar] [CrossRef]
- Xu, Z.; Zhao, R.; Huang, X.; Wang, X.; Tang, S. Fabrication and biocompatibility of agarose acetate nanofibrous membrane by electrospinning. Carbohydr. Polym. 2018, 197, 237–245. [Google Scholar] [CrossRef]
- Sanchez-Alvarado, D.I.; Guzmán-Pantoja, J.; Páramo-García, U.; Maciel-Cerda, A.; Martínez-Orozco, R.D.; Vera-Graziano, R. Morphological Study of Chitosan/Poly (Vinyl Alcohol) Nanofibers Prepared by Electrospinning, Collected on Reticulated Vitreous Carbon. Int. J. Mol. Sci. 2018, 19, 1718. [Google Scholar] [CrossRef] [Green Version]
- Biranje, S.; Madiwale, P.; Adivarekar, R.V. Electrospinning of chitosan/PVA nanofibrous membrane at ultralow solvent concentration. J. Polym. Res. 2017, 24, 92. [Google Scholar] [CrossRef]
- Abdal-Hay, A.; Hamdy, A.S.; Khalil, K.A. Fabrication of durable high performance hybrid nanofiber scaffolds for bone tissue regeneration using a novel, simple in situ deposition approach of polyvinyl alcohol on electrospun nylon 6 nanofibers. Mater. Lett. 2015, 147, 25–28. [Google Scholar] [CrossRef]
- Xu, T.; Yao, Q.; Miszuk, J.M.; Sanyour, H.J.; Hong, Z.; Sun, H.; Fong, H. Tailoring weight ration of PCL/PLA in electrospun three-dimensional nanofibrous scaffolds and the effect on osteogenic differentiation of stem cells. Colloids Surf. B 2018, 171, 31–39. [Google Scholar] [CrossRef]
- Ranganathan, S.; Balagangadharan, K.; Selvamurugan, N. Chitosan and gelatin-based electrospun fibers for bone tissue engineering. Int. J. Biol. Macromol. 2019, 133, 354–364. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, J.; Li, P.; Ning, N. The Effect of Chitosan in Wound Healing: A Systematic Review. Adv. Skin Wound Care 2020, 34, 262–266. [Google Scholar] [CrossRef]
- Salazar-Brann, S.A.; Patiño-Herrera, R.; Navarrete-Damián, J.; Louvier-Hernández, J.F. Electrospinning of chitosan from different acid solutions. AIMS Bioeng. 2021, 8, 112–129. [Google Scholar] [CrossRef]
- Qasim, S.B.; Zafar, M.S.; Najeeb, S.; Khurshid, Z.; Shah, A.H.; Husain, S.; Rehman, I.U. Electrospinning of chitosan-based solutions for tissue engineering and regenerative medicine. Int. J. Mol. Sci. 2018, 19, 407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mengistu Lemma, S.; Bossard, F.; Rinaudo, M. Preparation of pure and stable chitosan nanofibers by electrospinning in the presence of poly(ethylene oxide). Int. J. Mol. Sci. 2016, 17, 1790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirzaeei, S.; Taghe, S.; Asare-Addo, K.; Nokhodchi, A. Polyvinyl Alcohol/Chitosan Single-Layered and Polyvinyl Alcohol/Chitosan/Eudragit RL100 Multi-layered Electrospun Nanofibers as an Ocular Matrix for the Controlled Release of Ofloxacin: An In Vitro and In Vivo Evaluation. AAPS PharmSciTech 2021, 22, 170–183. [Google Scholar] [CrossRef] [PubMed]
- Paipitak, K.; Pornpra, T.; Mongkontalang, P.; Techitdheer, W.; Pecharapa, W. Characterization of PVA-Chitosan Nanofibers Prepared by Electrospinning. Procedia Eng. 2011, 8, 101–105. [Google Scholar] [CrossRef] [Green Version]
- Dong, Z.; Yuan, Q.; Huang, K.; Xu, W.; Liu, G.; Gu, Z. Gelatin methacryloyl (GelMA)-based biomaterials for bone regeneration. RCS Adv. 2019, 9, 17737–17744. [Google Scholar] [CrossRef] [Green Version]
- Mamidi, N.; Velasco Delgadillo, R.M.; Barrera, E.V. Covalently functionalized carbon nano-onions integrated gelatin methacryloyl nanocomposite hydrogel containing γ-cyclodextrin as drug carrier for high performance pH-triggered drug release. Pharmaceuticals 2021, 14, 291. [Google Scholar] [CrossRef]
- Rico-Llanos, G.A.; Borrego-Gonsalez, S.; Moncayo-Donoso, M.; Becerra, J.; Visser, R. Collagen type 1 biomaterials as scaffolds for bone tissue engineering. Polymers 2021, 13, 599. [Google Scholar] [CrossRef]
- Sell, S.A.; McClure, M.J.; Garg, K.; Wolfe, P.S.; Bowlin, G.L. Electrospinning of collagen/biopolymers for regenerative medicine and cardiovascular tissue engineering. Adv. Drud. Deliv. Rev. 2009, 61, 1007–1019. [Google Scholar] [CrossRef]
- Blackstone, B.N.; Gallentine, S.C.; Powell, H.M. Collagen-based electrospun materials for tissue engineering: A systematic review. Bioengineering 2021, 8, 39. [Google Scholar] [CrossRef]
- Park, W.H.; Jeong, L.; Yoo, D.I.; Hudson, S. Effect of chitosan on morphology and conformation of silk fibroin. Polymer 2004, 45, 7151–7157. [Google Scholar] [CrossRef]
- Chen, J.-P.; Chen, S.H.; Lai, G.J. Preparation and characterization of biomimetic silk fibroin/chitosan composite nanofibers by electrospinning for osteoblasts culture. Nanoscale Res. Lett. 2012, 7, 170–181. [Google Scholar] [CrossRef] [Green Version]
- Mamidi, N.; Delgadillo, R.M.V. Design, fabrication and drug release potential of dual stimuli-responsive composite hydrogel nanoparticle interfaces. Colloids Surf. B Biointerfaces 2021, 204, 111819. [Google Scholar] [CrossRef] [PubMed]
- Siquaira, L.; Ribeiro, N.; Paredes, M.B.A.; Grenho, L.; Cunha-Reis, C.; Trichês, E.S.; Fernandes, M.H.; Sousa, S.R.; Monteiro, F.J. Influence of PLLA/PCL/HA scaffold fiber orientation on mechanical properties and osteoblast behavior. Materials 2019, 12, 3879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwak, S.; Haider, A.; Gupta, K.C.; Kim, S.; Kang, I.K. Micro/Nano multilayered scaffolds of PLGA and collagen by alternately electrospinning for bone tissue engi-neering. Nanoscale Res. Lett. 2016, 11, 323–339. [Google Scholar] [CrossRef] [Green Version]
- Siddiqui, N.; Ba Linh, N.-T.; Yang, H.M.; Lee, B.T. Electrospun polycaprolactone fibers in bone tissue engineering: A review. Mol. Biotechnol. 2021, 63, 363–388. [Google Scholar] [CrossRef] [PubMed]
- Abdal-hay, A.; Pant, H.R.; Lim, J.K. Super-hydrophilic electrospun nylon-6/hydroxyapatite membrane for bone tissue engineering. Eur. Polym. J. 2013, 49, 1314–1321. [Google Scholar] [CrossRef]
- Fu, Y.; Liu, L.; Cheng, R.; Cui, W. ECM Decorated Electrospun Nanofiber for Improving Bone Tissue Regeneration. Polymers 2018, 10, 272. [Google Scholar] [CrossRef] [Green Version]
- Gao, S.; Guo, W.; Chen, M.; Yuan, Z.; Wang, M.; Zhang, Y.; Liu, S.; Xi, T.; Guo, Q. Fabrication and characterization of electrospun nanofibers composed of decellularized meniscus extracellular matrix and polycaprolactone for meniscus tissue engineering. J. Mater. Chem. B 2017, 5, 2273–2285. [Google Scholar] [CrossRef]
- Park, J.-Y.; Lee, J.H.; Kim, C.H.; Kim, Y.J. Fabrication of polytetrafluoroethylene nanofibrous membranes for guided bone regeneration. RCS Adv. 2018, 8, 34359–34369. [Google Scholar] [CrossRef] [Green Version]
- Mamidi, N.; Zuníga, A.E.; Villela-Castrejón, J. Engineering and evaluation of forcespun functionalized carbon nano-onions reinforced poly (ε-caprolactone) composite nanofibers for pH-responsive drug release. Mater. Sci. Eng. C 2020, 112, 110928. [Google Scholar] [CrossRef]
- Metwally, S.; Ferraris, S.; Spriano, S.; Krysiak, Z.J.; Kaniuk, Ł.; Marzec, M.M.; Kim, S.K.; Szewczyk, P.K.; Gruszczyński, A.; Wytrwal-Sarna, M.; et al. Surface potential and roughness controlled cell adhesion and collagen formation in electrospun PCL fibers for bone regeneration. Mater. Des. 2020, 194, 108915. [Google Scholar] [CrossRef]
- Shapourzadeh, A.; Atyabi, S.M.; Irani, S.; Bakhshi, H. Enhanced adipose mesenchymal stem cells proliferation by carboxymethyl-chitosan functionalized polycaprolactone nanofiber. Iran. Biomed. J. 2020, 24, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Zhao, H.; Muhammad, H.; Dong, M.; Besenbacher, F.; Chen, M. Dual-delivery of FGF-2/CTGF from Silk Fibroin/PLCL-PEO Coaxial Fibers Enhances MSC Proliferation and Fi-brogenesis. Sci. Rep. 2017, 7, 8509. [Google Scholar] [CrossRef] [PubMed]
- Kan, Y.; Salimon, A.I.; Korsunsky, A.M. On the electrospinning of nanostructured collagen-PVA fiber mats. Mater. Today Proc. 2020, 33, 2013–2019. [Google Scholar] [CrossRef]
- Sharifi, F.; Atyabi, S.M.; Norouzian, D.; Zandi, M.; Irani, S.; Bakhshi, H. Polycaprolactone/carboxymethyl chitosan nanofibrous scaffolds for bone tissue engineering application. Int. J. Biol. Macromol. 2018, 115, 243–248. [Google Scholar] [CrossRef]
- Fathi, A.; Khanmohammadi, M.; Goodarzi, A.; Foroutani, L.; Mobarakeh, Z.T.; Saremi, J.; Arabpour, Z.; Ai, J. Fabrication of chitosan-polyvinyl alcohol and silk electrospun fiber seeded with differentiated keratinocyte for skin tissue regeneration in animal wound model. J. Biol. Eng. 2020, 14, 1–14. [Google Scholar] [CrossRef]
- Junka, R.; Yu, X. Polymeric nanofibrous scaffolds laden with cell-derived extracellular matrix for bone regeneration. Mater. Sci. Eng. C 2020, 113, 110981. [Google Scholar] [CrossRef]
- Dong, C.; Qiao, F.; Chen, G.; Lv, Y. Demineralized and decellularized bone extracellular matrix-incorporated electrospun nanofibrous scaffold for bone regeneration. J. Mater. Chem. B 2021, 9, 6881–6894. [Google Scholar] [CrossRef]
- Carvalho, M.; Silva, J.C.; Udangawa, R.N.; Cabral, J.M.S.; Ferreira, F.C.; da Silva, C.L.; Linhardt, R.J.; Vashishth, D. Co-culture cell-derived extracellular matrix loaded electrospun microfibrous scaffolds for bone tissue en-gineering. Mater. Sci. Eng. C 2019, 99, 479–490. [Google Scholar] [CrossRef]
- Jiwoon, J.; Jun, H.K.; Jung, H.S.; Nathaniel, S.H.; Chan, Y.H. Bioactive calcium phosphate materials and applications in bone regeneration. Biomater. Res. 2019, 23, 4. [Google Scholar] [CrossRef] [Green Version]
- Bao, M.; Wang, X.; Yuan, H.; Lou, X.; Zhao, Q.; Zhang, Y. HAp incorporated ultrafine polymeric fibers with shape memory effect for potential use in bone screw hole healing. J. Mater. Chem. B 2016, 4, 5308–5320. [Google Scholar] [CrossRef] [PubMed]
- Kundu, K.; Afshar, A.; Katti, D.R.; Edirisinghe, M.; Katti, K.S. Composite nanoclay-hydroxyapatite-polymer fiber scaffolds for bone tissue engineering manufactured using pressurized gyration. Compos. Sci. Technol. 2021, 202, 108598. [Google Scholar] [CrossRef]
- Kosowska, K.; Domalik-Pyzik, P.; Krok-Borkowicz, M.; Chłopek, J. Polylactide/Hydroxyapatite Nonwovens Incorporated into Chitosan/Graphene Materials Hydrogels to Form Novel Hierarchical Scaffolds. Int. J. Mol. Sci. 2020, 21, 2330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadat-Shojai, M.; Khorasani, M.-T.; Jamshidi, A. A new strategy for fabrication of bone scaffolds using electrospun nano-HAp/PHB fibers and protein hydrogels. Chem. Eng. J. 2016, 289, 38–47. [Google Scholar] [CrossRef]
- Haj, J.; Khalil, T.H.; Falah, M.; Zussman, E.; Srouji, S. An ECM-Mimicking, Mesenchymal Stem Cell-Embedded Hybrid Scaffold for Bone Regeneration. BioMed Res. Int. 2017, 2017, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Vozzi, G.; Corallo, C.; Carta, S.; Fortina, M.; Gattazzo, F.; Galletti, M.; Giordano, N. Collagen-gelatin-genipin-hydroxyapatite composite scaffolds colonized by human primary osteoblasts are suitable for bone tissue engineering applications: In vitro evidences. J. Biomed. Mater. Res. A 2014, 102, 1415–1421. [Google Scholar] [CrossRef]
- Shaban, N.Z.; Kenawy, M.Y.; Taha, N.A.; Abd El-Latif, M.M.; Ghareeb, D.A. Cellulose acetate nanofibers: Incorporating hydroxyapatite (HA), HA/berberine or HA/moghat composites, as scaffolds to enhance in vitro osteoporotic bone regeneration. Polymers 2021, 13, 4140. [Google Scholar] [CrossRef]
- Karbovniczek, J.E.; Kaniuk, Ł.; Berniak, K.; Gruszczyński, A.; Stachewicz, U. Enhanced cells anchoring to electrospun hybrid scaffolds with PHBV and HA particles for bone tissue regeneration. Front. Bioeng. Biotechnol. 2021, 9, 632029. [Google Scholar] [CrossRef]
- Zhang, X.; Meng, S.; Huang, Y.; Xu, M.; He, Y.; Lin, H.; Han, J.; Chai, Y.; Wei, Y.; Deng, X. Electrospun Gelatin/β-TCP Composite Nanofibers Enhance Osteogenic Differentiation of BMSCs andIn VivoBone Formation by Activating Ca2+-Sensing Receptor Signaling. Stem Cells Int. 2015, 2015, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Terzioglu, P. Electrospun chitosan/gelatin/nano-CaCO3 hybrid nanofibers for potential tissue engineering applications. J. Nat. Fibers 2021, 8, 1207–1216. [Google Scholar] [CrossRef]
- Peranidze, K.K.; Safronova, T.; Kil’Deeva, N.; Chernogortseva, M.; Selezneva, I.; Shatalova, T.; Rau, J. Biocompatible composite films and fibers based on Poly(Vinyl alcohol) and powders of calcium salts. Smart Mater. Med. 2021, 2, 292–301. [Google Scholar] [CrossRef]
- Nagarajan, S.; Soussan, L.; Bechelany, M.; Teyssier, C.; Cavaillès, V.; Pochat-Bohatier, C.; Miele, P.; Kalkura, N.; Janot, J.-M.; Balme, S. Novel biocompatible electrospun gelatin fiber mats with antibiotic drug delivery properties. J. Mater. Chem. B 2015, 4, 1134–1141. [Google Scholar] [CrossRef]
- Pati, F.; Adhikari, B.; Dhara, S. Collagen Intermingled Chitosan-Tripolyphosphate Nano/Micro Fibrous Scaffolds for Tissue-Engineering Application. Biomater. Sci. 2012, 23, 1923–1938. [Google Scholar] [CrossRef] [PubMed]
- Elkhouly, H.; Mamdouh, W.; El-Korashy, D.I. Electrospun nano-fibrous bilayer scaffold prepared from polycaprolactone/gelatin and bioactive glass for bone tissue engineering. J. Mater. Sci. Mater. Med. 2021, 32, 111–126. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef] [PubMed]
- Maji, K.; Pramanik, K. Electrospun scaffold for bone regeneration. Int. J. Polym. Mater. 2021, 1–18. [Google Scholar] [CrossRef]
- Drag, M. Numerical infeasibilities of nanofibrous mats process design. Appl. Sci. 2021, 11, 11488. [Google Scholar] [CrossRef]
- Begum, H.A.; Khan, K.R. Study on the various types of needle based and needleless electrospinning system for nanofiber pro-duction. Int. J. Text Sci. 2017, 6, 110–117. [Google Scholar]
- Huang, B.; Aslan, E.; Jiang, Z.; Daskalakis, E.; Jiao, M.; Aldalbahi, A.; Vyas, C.; Bártolo, P. Engineered Dual-Scale Poly (e-Caprolactone) Scaffolds Using 3D Printing and Rotational Electrospinning for Bone Tissue Regeneration. Addit. Manuf. 2020, 36, 101452. [Google Scholar] [CrossRef]
- Partheniadis, I.; Nikolakakis, I.; Laidmäe, I.; Heinämäki, J. A Mini-Review: Needleless Electrospinning of Nanofibers for Pharmaceutical and Biomedical Applications. Processes 2020, 8, 673. [Google Scholar] [CrossRef]
- Su, Y.; Su, Q.; Liu, W.; Lim, M.; Venugopal, J.R.; Mo, X.; Ramakrishna, S.; Al-Deyab, S.S.; El-Newehy, M. Controlled release of bone morphogenetic protein 2 and dexamethasone loaded in core–shell PLLACL–collagen fibers for use in bone tissue engineering. Acta Biomater. 2012, 8, 763–771. [Google Scholar] [CrossRef]
- Udomluck, N.; Koh, W.-G.; Lim, D.-J.; Park, H. Recent Developments in Nanofiber Fabrication and Modification for Bone Tissue Engineering. Int. J. Mol. Sci. 2020, 21, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asran, A.S.; Henning, S.; Michler, G.H. Polyvinyl Alcohol–Collagen–Hydroxyapatite Biocomposite Nanofibrous Scaffold: Mimicking the Key Features of Natural Bone at the Nanoscale Level. Polymer 2010, 51, 868–876. [Google Scholar] [CrossRef]
- Nam, J.; Huang, Y.; Agarwal, S.; Lannutti, J. Improved Cellular Infiltration in Electrospun Fiber via Engineered Porosity. Tissue Eng. 2007, 13, 2249–2257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, Y.; Xu, L.; Wang, M. High-Throughput Preparation of Silk Fibroin Nanofibers by Modified Bubble-Electrospinning. Nanomaterials 2018, 8, 471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khorshidi, S.; Solouk, A.; Mirzadeh, H.; Mazinani, S.; Lagaron, J.M.; Sharifi, S.; Ramakrishna, S. A review of key challenges of electrospun scaffolds for tissue-engineering applications. J. Tissue Eng. Regen. Med. 2016, 10, 715–738. [Google Scholar] [CrossRef]
- Mamidi, N.; Delgadillo, R.M.V.; Castrejón, J.V. Unconventional and facile production of a stimuli-responsive multifunctional system for simultaneous drug delivery and environmental remediation. Environ. Sci. Nano 2021, 8, 2081–2097. [Google Scholar] [CrossRef]
- Mamidi, N.; Romo, I.L.; Barrera, E.V.; Elías-Zúñiga, A. High throughput fabrication of curcumin embedded gelatin-polylactic acid forcespun fiber-aligned scaffolds for the controlled release of curcumin. MRS Commun. 2018, 8, 1395–1403. [Google Scholar] [CrossRef]





| Polymer Type | Suitable Composite | Feature | |
|---|---|---|---|
| Natural polymers | Collagen | collagen/cellulose [24], PCL/collagen [25], collagen/PLGA [26] | Significant component of native ECM, low cytotoxic response, weak mechanical properties, high degradation rate |
| Silk fibroin | silk fibroin/chitosan [27], silk fibroin/PVA, silk fibroin/PLA, silk fibroin/PEO [28] | Sufficient biocompatibility, strong mechanical properties, low degradation, easy to process, no immunogenic response in vivo | |
| Gelatin | chitosan/gelatin [29], gelatin/PEO [30], gelatin/PCL [31], gelatin/silk fibroin [15] | Similar to collagen in structure, relatively high tensile modulus, suitable biocompatibility, highly affordable | |
| Chitosan | silk fibroin/chitosan [27], chitosan/gelatin [29], chitosan/agarose [32,33], chitosan/PVA [34,35], chitosan/PEO [35] | Suitable biocompatibility, strong fibers in combination with PVA, requires toxic acidic agents for electrospinning | |
| Cellulose | collagen/cellulose [24] | Significant biocompatibility, weak mechanical properties, high degradation rate | |
| Synthetic polymers | N6 | N6/PVA [36] | Sufficient biocompatibility, controllable conformation, enhanced wettability resulting in good MC3T3-E1 cell attachment for N6/PVA |
| PCL | PCL/collagen [25], PCL/PLA [37] | Sufficient biocompatibility and biodegradability, highly affordable, increased hydrophobicity resulting in poor cell attachment | |
| PLA | PCL/PLA [37] | Sufficient biocompatibility, improved mechanical properties compare to analogs, low degradation, inflammatory reactions caused by its by-product | |
| PLGA | collagen/PLGA [26] | Sufficient biocompatibility, high degradation rate compared to PLA | |
| PEO | gelatin/PEO [30], silk fibroin/PEO [28], chitosan/PEO [35] | Sufficient biocompatibility, mainly used as additive to improve properties of the artificial ECM | |
| PVA | silk fibroin/PVA [28], chitosan/PVA [34,35], N6/PVA [36] | Suitable biocompatibility, mainly used as additive to improve properties of the artificial ECM, highly affordable, process with various hydrolysis degrees, high degradation rate | |
| Electrospinning type | Needle-based (capillary) | Multiaxial electrospinning: -coaxial -triaxial |
| Bi-component electrospinning | ||
| Multineddle electrospinning | ||
| Electroblowing/Gas-assisted/Gas jet electrospinning | ||
| Magnetic field assisted electrospinning | ||
| Conjugate electrospinning | ||
| Centrifugal electrospinning | ||
| Needleless (capillary-free) | Bubble electrospinning | |
| Two-layer fluid electrospinning | ||
| Splashing electrospinning | ||
| Melt differential electrospinning | ||
| Gas-assisted melt differential electrospinning | ||
| Rotary cone electrospinning | ||
| Rotating roller electrospinning/Nanospider technology | ||
| Edge electrospinning | ||
| Blown bubble electrospinning |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peranidze, K.; Safronova, T.V.; Kildeeva, N.R. Fibrous Polymer-Based Composites Obtained by Electrospinning for Bone Tissue Engineering. Polymers 2022, 14, 96. https://doi.org/10.3390/polym14010096
Peranidze K, Safronova TV, Kildeeva NR. Fibrous Polymer-Based Composites Obtained by Electrospinning for Bone Tissue Engineering. Polymers. 2022; 14(1):96. https://doi.org/10.3390/polym14010096
Chicago/Turabian StylePeranidze, Kristina, Tatiana V. Safronova, and Nataliya R. Kildeeva. 2022. "Fibrous Polymer-Based Composites Obtained by Electrospinning for Bone Tissue Engineering" Polymers 14, no. 1: 96. https://doi.org/10.3390/polym14010096
APA StylePeranidze, K., Safronova, T. V., & Kildeeva, N. R. (2022). Fibrous Polymer-Based Composites Obtained by Electrospinning for Bone Tissue Engineering. Polymers, 14(1), 96. https://doi.org/10.3390/polym14010096