Crosslinked 4-Vinylpyridine Monodisperse Functional Microspheres for Sorption of Ibuprofen and Ketoprofen
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Poly(4VP-co-14DMB) and Poly(4VP-co-TRIM) Copolymers
2.3. Methods of Analysis
3. Results
3.1. Characterization of Copolymers
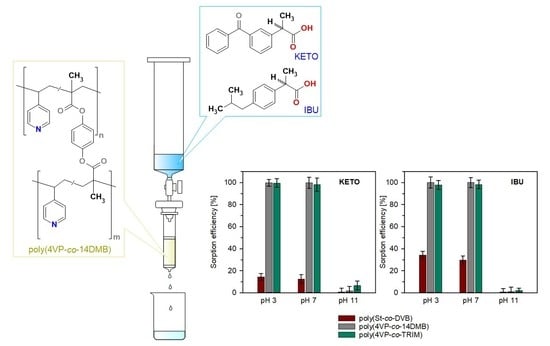
3.2. Sorption Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rao, P.; Knaus, E.E. Evolution of nonsteroidal anti-inflammatory drugs (NSAIDs): Cyclooxygenase (COX) inhibition and beyond. J. Pharm. Pharm. Sci. 2008, 20, 81–110. [Google Scholar] [CrossRef] [Green Version]
- Oleszkiewicz, P.; Krysinski, J.; Religioni, U.; Merks, P. Access to Medicines via Non-Pharmacy Outlets in European Countries—A Review of Regulations and the Influence on the Self-Medication Phenomenon. Healthcare 2021, 9, 123. [Google Scholar] [CrossRef]
- Available online: https://www.globenewswire.com/news-release/2022/01/26/2373168/0/en/Ibuprofen-Market-and-Ibuprofen-API-Market-2022-Extensive-Research-By-Latest-Industry-Innovations-Size-Share-Recent-Developments-Market-Position-Growth-Drivers-Business-Opportunity-.html (accessed on 10 April 2022).
- Tambosi, J.L.; Yamanaka, L.Y.; José, H.J.; Moreira, R.; Schröder, H.F. Recent research data on the removal of pharmaceuticals from sewage treatment plants (STP). Quím. Nova 2010, 33, 411–420. [Google Scholar] [CrossRef]
- Carmona, E.; Andreu, V.; Pico, Y. Occurrence of acid pharmaceuticals and personal care products in Turia River Basis: From waste to drinking water. Sci. Total Environ. 2014, 484, 53–63. [Google Scholar] [CrossRef]
- Kermia, A.E.B.; Fouial-Djebbaret, D.; Trari, M. Occurrence, fate and removal efficiencies of pharmaceuticals in wastewater treatment plants (WWTPs) discharging in the coastal environment of Algiers. Comptes Rendus Chim. 2016, 19, 963–970. [Google Scholar] [CrossRef]
- Santos, J.L.; Aparicio, I.; Alonso, E.; Callejon, M. Simultaneous determination of pharmaceutically active compounds in wastewater samples by solid phase extraction and high-performance liquid chromatography with diode array and fluorescence detectors. Anal. Chim. Acta 2005, 550, 116–122. [Google Scholar] [CrossRef]
- Chopra, S.; Kumar, D. Ibuprofen as an emerging organic contaminant in environment, distribution and remediation. Heliyon 2020, 6, e04087. [Google Scholar] [CrossRef]
- Zuccato, E.; Castiglioni, S.; Fanelli, R. Identification of the pharmaceuticals for human use contaminating the Italian aquatic environment. J. Hard Mater. 2005, 122, 205–209. [Google Scholar] [CrossRef]
- Togola, A.; Budzinski, H. Analytical development for analysis of pharmaceuticals in water samples by SPE and GC–MS. Anal. Bioanal. Chem. 2007, 388, 627–635. [Google Scholar] [CrossRef]
- Lee, H.B.; Peart, T.E.; Svoboda, M.L. Determination of endocrine-disrupting phenols, acidic pharmaceuticals, and personal-care products in sewage by solid-phase extraction and gas chromatography-mass spectrometry. J. Chromatogr. A 2005, 1094, 122–129. [Google Scholar] [CrossRef]
- Patrolecco, L.; Ademollo, N.; Grenni, P.; Tolomei, A.; Caracciolo, A.B.; Capri, S. Simultaneous determination of human pharmaceuticals in water samples by solid phase extraction and HPLC with UV-fluorescence detection. Microchem. J. 2013, 107, 165–171. [Google Scholar] [CrossRef]
- Gomez, M.J.; Petrovic, M.; Fernandez-Alba, A.R.; Barcelo, D. Determination of pharmaceuticals of various therapeutic classes by solid-phase extraction and liquid chromatography–tandem mass spectrometry analysis in hospital effluent wastewaters. J. Chromatogr. A 2006, 1114, 224–233. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Q.; Darisaw, S.; Ehie, O.; Wang, G. Simultaneous quantification of polycyclic aromatic hydrocarbons (PAHs), polychlorinated biphenyls (PCBs), and pharmaceuticals and personal care products (PPCPs) in Mississippi river water, in New Orleans, Louisiana, USA. Chemosphere 2007, 66, 1057–1069. [Google Scholar] [CrossRef]
- Hashim, N.H.; Khan, S.J. Enantioselective analysis of ibuprofen, ketoprofen and naproxen in wastewater and environmental water samples. J. Chromatogr. A 2011, 1218, 4746–4754. [Google Scholar] [CrossRef]
- Olcer, Y.A.; Demirkurt, M.; Demir, M.M.; Eroglu, A.E. Development of molecularly imprinted polymers (MIPs) as a solid phase extraction (SPE) sorbent for the determination of ibuprofen in water. RSC Adv. 2017, 7, 31441–31447. [Google Scholar] [CrossRef] [Green Version]
- Madikizela, L.M.; Chimuka, L. Synthesis, adsorption and selectivity studies of a polymer imprinted with naproxen, ibuprofen and diclofenac. J. Environ. Chem. Eng. 2016, 4, 4029–4037. [Google Scholar] [CrossRef]
- Madikizela, L.M.; Chimuka, L. Determination of ibuprofen, naproxen and diclofenac in aqueoussamples using a multi-template molecularly imprinted polymer asselective adsorbent for solid-phase extraction. J. Pharm. Biomed. Anal. 2016, 128, 210–215. [Google Scholar] [CrossRef]
- Zunngu, S.S.; Madikizela, L.M.; Chimuka, L.; Mdluli, P.S. Synthesis and application of a molecularly imprinted polymer in the solid-phase extraction of ketoprofen from wastewater. Comptes Rendus Chim. 2017, 20, 585–591. [Google Scholar] [CrossRef]
- Gholivand, M.B.; Karimian, N.; Malekzadeh, G. Computational design and synthesis of a high selective molecularly imprinted polymer for voltammetric sensing of propazine in food samples. Talanta 2012, 89, 513–520. [Google Scholar] [CrossRef]
- Grochowicz, M.; Gawdzik, B. Preparation and characterization of porous crosslinked microspheres of new aromatic methacrylates. J. Porous Mat. 2013, 20, 339–349. [Google Scholar] [CrossRef] [Green Version]
- Grochowicz, M.; Szajnecki, Ł.; Gawdzik, B. 4VP-TRIM composite polymer particles and their application as adsorbents. Adsorpt. Sci. Technol. 2015, 33, 609–616. [Google Scholar] [CrossRef]
- Unsal, E.; Camli, S.T.; Senel, S.; Tuncel, A. Chromatographic performance of monodisperse–macroporous particles produced by modified seeded polymerization. I: Effect of monomer/seed latex ratio. J. Appl. Polym. Sci. 2004, 92, 607–618. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Halasz, I.; Martin, K. Bestimmung der Porenverteilung (10–4000 Å) von Festkörpern mit der Methode der Ausschluß-Chromatographie. Ber. Bunsen. Ges. Phys. Chem. 1975, 79, 731–732. [Google Scholar] [CrossRef]
- Nevejans, F.; Verzele, M. On the structure and chromatographic behaviour of polystyrene phases. J. Chromatogr. 1987, 406, 325–342. [Google Scholar] [CrossRef]
- Nevejans, F.; Verzele, M. Porous polystyrene packings: Characterization through pore structure determination. Chromatographia 1985, 20, 173–178. [Google Scholar] [CrossRef]
- Gawdzik, B. Studies on the porous structure of di(methacryloyloxymethyl) naphthalene-divinylbenzene copolymers by inverse exclusion chromatography. Chromatographia 1991, 31, 21–27. [Google Scholar] [CrossRef]
- Grochowicz, M. Poly(glycidyl methacrylate-co-1,4-dimethacryloyloxybenzene) monodisperse microspheres—Synthesis, characterization and application as chromatographic packings in RP-HPLC. React. Funct. Polym. 2019, 137, 1–10. [Google Scholar] [CrossRef]
- Cheng, C.M.; Vanderhoff, J.W.; El-Aasser, M.S. Monodisperse porous polymer particles: Formation of the porous structure. J. Polym. Sci. A Polym. Chem. 1992, 30, 245–256. [Google Scholar] [CrossRef]
- Smigol, V.; Svec, F. Synthesis and properties of uniform beads based on macroporous copolymer glycidyl methacrylate–ethylene dimethacrylate: A way to improve separation media for HPLC. J. Appl. Polym. Sci. 1992, 46, 1439–1448. [Google Scholar] [CrossRef]
- Rosenberg, J.E.; Flodin, P. Macroporous gels. 1. Polymerization of trimethylolpropane trimethacrylate in toluene. Macromolecules 1986, 19, 1543–1546. [Google Scholar] [CrossRef]
- Grochowicz, M.; Gawdzik, B.; Bartnicki, A. New tetrafunctional monomer 1,3-di(2-hydroxy-3-methacryloyloxypropoxy) benzene in the synthesis of porous microspheres. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 3190–3201. [Google Scholar] [CrossRef]
- Podkościelna, B.; Gawdzik, B.; Bartnicki, A. Use of a new methacrylic monomer, 4,4′-di(2-hydroxy-3-methacryloyloxypropoxy)benzophenone, in the synthesis of porous microspheres. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 7014–7026. [Google Scholar] [CrossRef]
- Davankov, V.A.; Tsyurupa, M.P. Structure and properties of hypercrosslinked polystyrene—the first representative of a new class of polymer networks. React. Polym. 1990, 13, 27–42. [Google Scholar] [CrossRef]
- Jaćkowska, M.; Bocian, S.; Gawdzik, B.; Grochowicz, M.; Buszewski, B. Influence of chemical modification on the porous structure of polymeric adsorbents. Mater. Chem. Phys. 2011, 130, 644–650. [Google Scholar] [CrossRef]
- Grochowicz, M. Investigation of the thermal behavior of 4-vinylpyridine-trimethylolpropane trimethacrylate copolymeric microspheres. J. Therm. Anal. Calorim. 2014, 118, 1603–1611. [Google Scholar] [CrossRef] [Green Version]
- Available online: https://go.drugbank.com/drugs/DB01050 (accessed on 10 April 2022).
- Available online: https://go.drugbank.com/drugs/DB01009 (accessed on 10 April 2022).
- Zhou, Y.; Cheng, G.; Chen, K.; Lu, J.; Lei, J.; Pu, S. Adsorptive removal of bisphenol A, chloroxylenol, and carbamazepine from water using a novel β-cyclodextrin polymer. Ecotoxicol. Environ. Saf. 2019, 170, 278–285. [Google Scholar] [CrossRef]










| Copolymer | %N | N2 Adsorption-Desorption | ISEC | ||||
|---|---|---|---|---|---|---|---|
| SBET [m2/g] | Vp [cm3/g] | PSDmax [nm] | Vp [cm3/g] | Vmicro [cm3/g] | PSDmax [nm] | ||
| poly(4VP-co-14DMB) | 5.56 | 89 | 0.26 | 4/50 | 0.81 | 0.12 | 1-2/17 |
| poly(4VP-co-TRIM) | 4.32 | 147 | 0.29 | 4/34 | 0.77 | 0.14 | 1-2/30 |
| poly(St-co-DVB) | - | 225 | 0.38 | 4/24 | 0.51 | 0.18 | 1-2/41 |
| Sample | T2% [°C] | T50% [°C] | Tmax1 [°C] | Δ m1 [%] | Tmax2 [°C] | Δ m2 [%] | Rm [%] |
|---|---|---|---|---|---|---|---|
| poly(4VP-co-14DMB) | 316 | 335 | 334 | 66.3 | 460 | 28.8 | 4.9 |
| poly(4VP-co-TRIM) | 301 | 322 | 320 | 70.7 | 469 | 25.8 | 3.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grochowicz, M.; Szajnecki, Ł.; Rogulska, M. Crosslinked 4-Vinylpyridine Monodisperse Functional Microspheres for Sorption of Ibuprofen and Ketoprofen. Polymers 2022, 14, 2080. https://doi.org/10.3390/polym14102080
Grochowicz M, Szajnecki Ł, Rogulska M. Crosslinked 4-Vinylpyridine Monodisperse Functional Microspheres for Sorption of Ibuprofen and Ketoprofen. Polymers. 2022; 14(10):2080. https://doi.org/10.3390/polym14102080
Chicago/Turabian StyleGrochowicz, Marta, Łukasz Szajnecki, and Magdalena Rogulska. 2022. "Crosslinked 4-Vinylpyridine Monodisperse Functional Microspheres for Sorption of Ibuprofen and Ketoprofen" Polymers 14, no. 10: 2080. https://doi.org/10.3390/polym14102080
APA StyleGrochowicz, M., Szajnecki, Ł., & Rogulska, M. (2022). Crosslinked 4-Vinylpyridine Monodisperse Functional Microspheres for Sorption of Ibuprofen and Ketoprofen. Polymers, 14(10), 2080. https://doi.org/10.3390/polym14102080