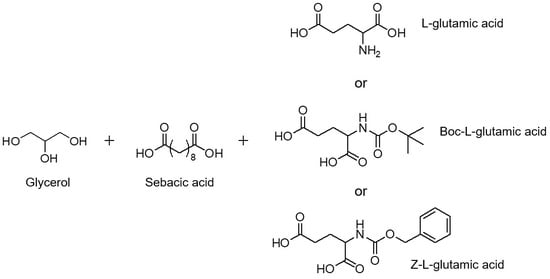
Incorporation of Glutamic Acid or Amino-Protected Glutamic Acid into Poly(Glycerol Sebacate): Synthesis and Characterization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis and Characterization of Prepolymers
2.3. Preparation of Elastomers
2.4. Fourier-Transform Infrared Spectroscopy
2.5. Thermal Properties
2.6. Mechanical Testing
2.7. Water Contact Angle Measurements
2.8. In Vitro Enzymatic Degradation
2.9. Cytocompatibility
2.10. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Langer, R.; Vacanti, J.P. Tissue Engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jagur-Grodzinski, J. Biomedical application of functional polymers. React. Funct. Polym. 1999, 39, 99–138. [Google Scholar] [CrossRef]
- Nair, L.S.; Laurencin, C.T. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. [Google Scholar] [CrossRef]
- Gunatillake, P.; Mayadunne, R.; Adhikari, R. Recent developments in biodegradable synthetic polymers. Biotechnol. Annu. Rev. 2006, 12, 301–347. [Google Scholar] [CrossRef]
- Wang, Y.D.; Ameer, G.A.; Sheppard, B.J.; Langer, R. A tough biodegradable elastomer. Nat. Biotechnol. 2002, 20, 602–606. [Google Scholar] [CrossRef]
- Yang, J.; Webb, A.R.; Ameer, G.A. Novel citric acid-based biodegradable elastomers for tissue engineering. Adv. Mater. 2004, 16, 511–516. [Google Scholar] [CrossRef]
- Crapo, P.M.; Wang, Y.D. Physiologic compliance in engineered small-diameter arterial constructs based on an elastomeric substrate. Biomaterials 2010, 31, 1626–1635. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.W.; Stolz, D.B.; Wang, Y.D. Substantial expression of mature elastin in arterial constructs. Proc. Natl. Acad. Sci. USA 2011, 108, 2705–2710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Q.Z.; Ishii, H.; Thouas, G.A.; Lyon, A.R.; Wright, J.S.; Blaker, J.J.; Chrzanowski, W.; Boccaccini, A.R.; Ali, N.N.; Knowles, J.C.; et al. An elastomeric patch derived from poly(glycerol sebacate) for delivery of embryonic stem cells to the heart. Biomaterials 2010, 31, 3885–3893. [Google Scholar] [CrossRef]
- Kemppainen, J.M.; Hollister, S.J. Tailoring the mechanical properties of 3D-designed poly(glycerol sebacate) scaffolds for cartilage applications. J. Biomed. Mater. Res. Part A 2010, 94a, 9–18. [Google Scholar] [CrossRef] [Green Version]
- Sundback, C.A.; Shyu, J.Y.; Wang, Y.D.; Faquin, W.C.; Langer, R.S.; Vacanti, J.P.; Hadlock, T.A. Biocompatibility analysis of poly(glycerol sebacate) as a nerve guide material. Biomaterials 2005, 26, 5454–5464. [Google Scholar] [CrossRef] [PubMed]
- Engelmayr, G.C.; Cheng, M.Y.; Bettinger, C.J.; Borenstein, J.T.; Langer, R.; Freed, L.E. Accordion-like honeycombs for tissue engineering of cardiac anisotropy. Nat. Mater. 2008, 7, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Hu, M.H.; Hu, J.J. Use of Aligned Microscale Sacrificial Fibers in Creating Biomimetic, Anisotropic Poly(glycerol sebacate) Scaffolds. Polymers 2019, 11, 1492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, C.N.; Lee, P.Y.; Tuan-Mu, H.Y.; Li, C.Y.; Hu, J.J. Fabrication of a mechanically anisotropic poly(glycerol sebacate) membrane for tissue engineering. J. Biomed. Mater. Res. B 2018, 106, 760–770. [Google Scholar] [CrossRef] [PubMed]
- Jeffries, E.M.; Allen, R.A.; Gao, J.; Pesce, M.; Wang, Y.D. Highly elastic and suturable electrospun poly(glycerol sebacate) fibrous scaffolds. Acta Biomater. 2015, 18, 30–39. [Google Scholar] [CrossRef] [Green Version]
- You, Z.R.; Hu, M.H.; Tuan-Mu, H.Y.; Hu, J.J. Fabrication of poly(glycerol sebacate) fibrous membranes by coaxial electrospinning: Influence of shell and core solutions. J. Mech. Behav. Biomed. Mater. 2016, 63, 220–231. [Google Scholar] [CrossRef]
- Wu, H.J.; Hu, M.H.; Tuan-Mu, H.Y.; Hu, J.J. Preparation of aligned poly(glycerol sebacate) fibrous membranes for anisotropic tissue engineering. Mater. Sci. Eng. C-Mater. Biol. Appl. 2019, 100, 30–37. [Google Scholar] [CrossRef]
- Ifkovits, J.L.; Devlin, J.J.; Eng, G.; Martens, T.P.; Vunjak-Novakovic, G.; Burdick, J.A. Biodegradable Fibrous Scaffolds with Tunable Properties Formed from Photo-Cross-Linkable Poly(glycerol sebacate). ACS Appl. Mater. Inter. 2009, 1, 1878–1886. [Google Scholar] [CrossRef] [Green Version]
- Lang, K.N.; Bhattacharya, S.; Ning, Z.Y.; Sanchez-Leija, R.J.; Bramson, M.T.K.; Centore, R.; Corr, D.T.; Linhardt, R.J.; Gross, R.A. Enzymatic Polymerization of Poly(glycerol-1,8-octanediol-sebacate): Versatile Poly(glycerol sebacate) Analogues that Form Monocomponent Biodegradable Fiber Scaffolds. Biomacromolecules 2020, 21, 3197–3206. [Google Scholar] [CrossRef]
- Ning, Z.Y.; Lang, K.N.; Xia, K.; Linhardt, R.J.; Gross, R.A. Lipase-Catalyzed Synthesis and Characterization of Poly(glycerol sebacate). Biomacromolecules 2022, 23, 398–408. [Google Scholar] [CrossRef]
- Rai, R.; Tallawi, M.; Grigore, A.; Boccaccini, A.R. Synthesis, properties and biomedical applications of poly(glycerol sebacate) (PGS): A review. Prog. Polym. Sci. 2012, 37, 1051–1078. [Google Scholar] [CrossRef]
- Loh, X.J.; Karim, A.A.; Owh, C. Poly(glycerol sebacate) biomaterial: Synthesis and biomedical applications. J. Mater. Chem. B 2015, 3, 7641–7652. [Google Scholar] [CrossRef] [PubMed]
- Vogt, L.; Ruther, F.; Salehi, S.; Boccaccini, A.R. Poly(Glycerol Sebacate) in Biomedical Applications-A Review of the Recent Literature. Adv. Healthc Mater. 2021, 10, 2002026. [Google Scholar] [CrossRef] [PubMed]
- Piszko, P.; Kryszak, B.; Piszko, A.; Szustakiewicz, K. Brief review on poly(glycerol sebacate) as an emerging polyester in biomedical application: Structure, properties and modifications. Polym. Med. 2021, 51, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.D.; Kim, Y.M.; Langer, R. In vivo degradation characteristics of poly(glycerol sebacate). J. Biomed. Mater. Res. Part A 2003, 66a, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.L.; Cook, W.D.; Thouas, G.A.; Chen, Q.Z. The mechanical characteristics and in vitro biocompatibility of poly(glycerol sebacate)-Bioglass (R) elastomeric composites. Biomaterials 2010, 31, 8516–8529. [Google Scholar] [CrossRef] [PubMed]
- Pomerantseva, I.; Krebs, N.; Hart, A.; Neville, C.M.; Huang, A.Y.; Sundback, C.A. Degradation behavior of poly(glycerol sebacate). J. Biomed. Mater. Res. Part A 2009, 91a, 1038–1047. [Google Scholar] [CrossRef]
- Sun, Z.J.; Wu, L.; Huang, W.; Zhang, X.L.; Lu, X.L.; Zheng, Y.F.; Yang, B.F.; Dong, D.L. The influence of lactic on the properties of Poly (glycerol-sebacate-lactic acid). Mater. Sci. Eng. C-Biomim. Supramol. Syst. 2009, 29, 178–182. [Google Scholar] [CrossRef]
- Sun, Z.J.; Wu, L.; Huang, W.; Chen, C.; Chen, Y.; Lu, X.L.; Zhang, X.L.; Yang, B.F.; Dong, D.L. Glycolic acid modulates the mechanical property and degradation of poly(glycerol, sebacate, glycolic acid). J. Biomed. Mater. Res. Part A 2010, 92a, 332–339. [Google Scholar] [CrossRef]
- Risley, B.B.; Ding, X.C.; Chen, Y.; Miller, P.G.; Wang, Y.D. Citrate Crosslinked Poly(Glycerol Sebacate) with Tunable Elastomeric Properties. Macromol. Biosci. 2021, 21, 2000301. [Google Scholar] [CrossRef]
- Liu, Q.Y.; Tan, T.W.; Weng, J.Y.; Zhang, L.Q. Study on the control of the compositions and properties of a biodegradable polyester elastomer. Biomed. Mater. 2009, 4, 025015. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.X.; Wu, H.W.; Wang, Z.H.; Zhang, J.J.; Zhu, J.; Ma, Y.F.; Yang, Z.G.; Yuan, Y. Optimized Synthesis of Biodegradable Elastomer PEGylated Poly(glycerol sebacate) and Their Biomedical Application. Polymers 2019, 11, 965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, A.; Gaharwar, A.K.; Iviglia, G.; Zhang, H.B.; Mukundan, S.; Mihaila, S.M.; Demarchi, D.; Khademhosseini, A. Highly elastomeric poly(glycerol sebacate)-co-poly(ethylene glycol) amphiphilic block copolymers. Biomaterials 2013, 34, 3970–3983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, W.F.; Li, T.; Xiang, S.F.; Ma, P.M.; Chen, M.Q. Influence of Glutamic Acid on the Properties of Poly(xylitol glutamate sebacate) Bioelastomer. Polymers 2013, 5, 1339–1351. [Google Scholar] [CrossRef]
- Wang, C.C.; Shih, T.Y.; Hsieh, Y.T.; Huang, J.L.; Wang, J. l-Arginine Grafted Poly(Glycerol Sebacate) Materials: An Antimicrobial Material for Wound Dressing. Polymers 2020, 12, 1457. [Google Scholar] [CrossRef]
- Singh, D.; Harding, A.J.; Albadawi, E.; Boissonade, F.M.; Haycock, J.W.; Claeyssens, F. Additive manufactured biodegradable poly(glycerol sebacate methacrylate) nerve guidance conduits. Acta Biomater. 2018, 78, 48–63. [Google Scholar] [CrossRef]
- Qazi, T.H.; Rai, R.; Dippold, D.; Roether, J.E.; Schubert, D.W.; Rosellini, E.; Barbani, N.; Boccaccini, A.R. Development and characterization of novel electrically conductive PANI-PGS composites for cardiac tissue engineering applications. Acta Biomater. 2014, 10, 2434–2445. [Google Scholar] [CrossRef]
- Winnacker, M.; Rieger, B. Poly(ester amide)s: Recent insights into synthesis, stability and biomedical applications. Polym. Chem. 2016, 7, 7039–7046. [Google Scholar] [CrossRef] [Green Version]
- Karimi, P.; Rizkalla, A.S.; Mequanint, K. Versatile Biodegradable Poly(ester amide)s Derived from alpha-Amino Acids for Vascular Tissue Engineering. Materials 2010, 3, 2346–2368. [Google Scholar] [CrossRef] [Green Version]
- Natarajan, J.; Madras, G.; Chatterjee, K. Poly(ester amide)s from Poly(ethylene terephthalate) Waste for Enhancing Bone Regeneration and Controlled Release. ACS Appl. Mater. Inter. 2017, 9, 28281–28297. [Google Scholar] [CrossRef]
- Hu, J.J.; Chen, G.W.; Liu, Y.C.; Hsu, S.S. Influence of Specimen Geometry on the Estimation of the Planar Biaxial Mechanical Properties of Cruciform Specimens. Exp. Mech. 2014, 54, 615–631. [Google Scholar] [CrossRef]
- Zhang, J.Z.; Xu, Y.H.; Cui, L.; Fu, A.P.; Yang, W.R.; Barrow, C.; Liu, J.Q. Mechanical properties of graphene films enhanced by homo-telechelic functionalized polymer fillers via pi-pi It stacking interactions. Compos. Part A Appl. Sci. Manuf. 2015, 71, 1–8. [Google Scholar] [CrossRef]
- Chen, T.; Li, M.X.; Liu, J.Q. pi-pi Stacking Interaction: A Nondestructive and Facile Means in Material Engineering for Bioapplications. Cryst. Growth Des. 2018, 18, 2765–2783. [Google Scholar] [CrossRef]
- Chen, Q.; Yang, X.; Li, Y. A comparative study on in vitro enzymatic degradation of poly(glycerol sebacate) and poly(xylitol sebacate). RSC Adv. 2012, 2, 4125. [Google Scholar] [CrossRef]
- Horrocks, R.; D’Souza, J.; Hamid, S.; Amin, M.; Maadhah, A. Handbook of Polymer Degradation; M. Dekker: New York, NY, USA, 1992. [Google Scholar]
- Krook, N.M.; Jaafar, I.H.; Sarkhosh, T.; LeBlon, C.; Coulter, J.P.; Jedlicka, S.S. In vitro examination of poly(glycerol sebacate) degradation kinetics: Effects of porosity and cure temperature. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 535–543. [Google Scholar] [CrossRef]










| Sample Code | Composition | Molar Ratio |
|---|---|---|
| PGS | glycerol: sebacic acid | 1: 1 |
| PGSE1 | glycerol: sebacic acid: L-glutamic acid | 1: 0.9: 0.1 |
| PGSE2 | glycerol: sebacic acid: L-glutamic acid | 1: 0.9: 0.2 |
| PGSE3 | glycerol: sebacic acid: L-glutamic acid | 1: 0.8: 0.2 |
| PGSE-B | glycerol: sebacic acid: Boc-L-glutamic acid | 1: 0.8: 0.2 |
| PGSE-Z | glycerol: sebacic acid: Z-L-glutamic acid | 1: 0.8: 0.2 |
| Sample Code | Molecular Weight and Its Distrubution (Mw/Mn/PDI) |
|---|---|
| PGS | 5261 / 2192 / 2.4 |
| PGSE1 | 5468 / 2497 / 2.2 |
| PGSE2 | 10177 / 3002 / 3.4 |
| PGSE3 | 5574 / 2362 / 2.4 |
| PGSE-B | 2243 / 1089 / 2.1 |
| PGSE-Z | 15604 / 2123 / 7.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Y.-S.; Hu, M.-H.; Jan, J.-S.; Hu, J.-J. Incorporation of Glutamic Acid or Amino-Protected Glutamic Acid into Poly(Glycerol Sebacate): Synthesis and Characterization. Polymers 2022, 14, 2206. https://doi.org/10.3390/polym14112206
Jiang Y-S, Hu M-H, Jan J-S, Hu J-J. Incorporation of Glutamic Acid or Amino-Protected Glutamic Acid into Poly(Glycerol Sebacate): Synthesis and Characterization. Polymers. 2022; 14(11):2206. https://doi.org/10.3390/polym14112206
Chicago/Turabian StyleJiang, Yi-Sheng, Ming-Hsien Hu, Jeng-Shiung Jan, and Jin-Jia Hu. 2022. "Incorporation of Glutamic Acid or Amino-Protected Glutamic Acid into Poly(Glycerol Sebacate): Synthesis and Characterization" Polymers 14, no. 11: 2206. https://doi.org/10.3390/polym14112206
APA StyleJiang, Y.-S., Hu, M.-H., Jan, J.-S., & Hu, J.-J. (2022). Incorporation of Glutamic Acid or Amino-Protected Glutamic Acid into Poly(Glycerol Sebacate): Synthesis and Characterization. Polymers, 14(11), 2206. https://doi.org/10.3390/polym14112206