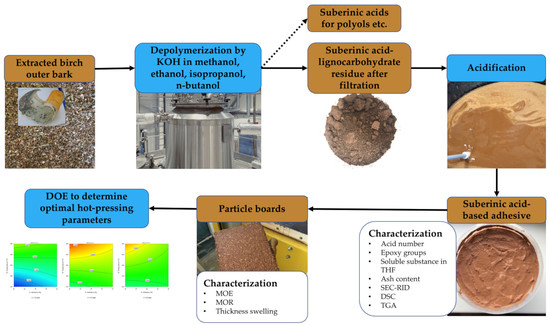
Utilization of Suberinic Acids Containing Residue as an Adhesive for Particle Boards
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material—Birch Outer Bark
2.2. Other Materials and Chemicals
2.3. Obtaining SA-Based Adhesive
2.4. Characterization of SA-Based Adhesive
2.4.1. Acid Number
2.4.2. Epoxy Groups
2.4.3. Soluble Substance in THF
2.4.4. Ash Content
2.4.5. Size-Exclusion Chromatography
2.4.6. Differential Scanning Calorimetry (DSC)
2.4.7. Thermogravimetric Analysis (TGA)
2.4.8. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
2.5. Particle Board Preparation
2.5.1. Comparison of Particle Board Properties Depending on SA-Based Adhesive Sample
2.5.2. Experimental Design to Determine Optimal Hot-Pressing Parameters
2.6. Evaluation of the Particle Board properties
3. Results and Discussion
3.1. Characterization of SA-Based Adhesive Samples
3.1.1. Acid Number, Epoxy Group Content, Soluble Substance in THF, Ash Content
3.1.2. Size-Exclusion Chromatography
3.1.3. DSC Analysis
3.1.4. TGA Analysis
3.1.5. FTIR Analysis
3.2. Choosing the Most Suitable Adhesive for PB Hot-Pressing
3.3. Experimental Design for Obtaining Particle Boards
3.3.1. MOE of the Particle Boards
3.3.2. MOR of the Particle Boards
3.3.3. TS 24 h of the Particle Boards
3.3.4. Particle Board Density
3.3.5. Determination of Optimal Parameters for Particle Board Hot-Pressing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vedernikov, D.N.; Shabanova, N.Y.; Roshchin, V.I. Change in the Chemical Composition of the Crust and Inner Bark of the Betula Pendula Roth. Birch (Betulaceae) with Tree Height. Russ. J. Bioorganic Chem. 2011, 37, 877–882. [Google Scholar] [CrossRef]
- Heinämäki, J.; Pirttimaa, M.M.; Alakurtti, S.; Pitkänen, H.P.; Kanerva, H.; Hulkko, J.; Paaver, U.; Aruväli, J.; Yliruusi, J.; Kogermann, K. Suberin Fatty Acids from Outer Birch Bark: Isolation and Physical Material Characterization. J. Nat. Prod. 2017, 80, 916–924. [Google Scholar] [CrossRef] [PubMed]
- Rizhikovs, J.; Zandersons, J.; Dobele, G.; Paze, A. Isolation of Triterpene-Rich Extracts from Outer Birch Bark by Hot Water and Alkaline Pre-Treatment or the Appropriate Choice of Solvents. Ind. Crops Prod. 2015, 76, 209–214. [Google Scholar] [CrossRef]
- Krasutsky, P.A.; Kolomitsyn, I.V.; Krasutskyy, D.A. Depolymerization Extraction of Compounds from Birch Bark. 2012. Available online: https://patents.google.com/patent/US8197870B2/en (accessed on 20 April 2022).
- Koptelova, E.N.; Kutakova, N.A.; Tret’yakov, S.I. Isolation of the Extractives and Betulin from Birch Bark Exposed to a Microwave Field. Russ. J. Bioorganic Chem. 2014, 40, 791–795. [Google Scholar] [CrossRef]
- Rižikovs, J.; Zandersons, J.; Paže, A.; Tardenaka, A.; Spince, B. Isolation of Suberinic Acids from Extracted Outer Birch Bark Depending on the Application Purposes. Balt. For. 2014, 20, 98–105. [Google Scholar]
- Graça, J. Suberin: The Biopolyester at the Frontier of Plants. Front. Chem. 2015, 3, 1–11. [Google Scholar] [CrossRef]
- Paze, A.; Rizhikovs, J.; Godiņa, D.; Makars, R.; Berzins, R. Development of Plywood Binder by Partial Replacement of Phenol-Formaldehyde Resins with Birch Outer Bark Components. Key Eng. Mater. 2021, 903, 229–234. [Google Scholar] [CrossRef]
- Tupciauskas, R.; Rizhikovs, J.; Grinins, J.; Paze, A.; Andzs, M.; Brazdausks, P.; Puke, M.; Plavniece, A. Investigation of Suberinic Acids-Bonded Particleboard. Eur. Polym. J. 2019, 113, 176–182. [Google Scholar] [CrossRef]
- Paze, A.; Rizhikovs, J. Study of an Appropriate Suberinic Acids Binder for Manufacturing of Plywood. Key Eng. Mater. 2019, 800, 251–255. [Google Scholar] [CrossRef]
- Pollard, M.; Beisson, F.; Li, Y.; Ohlrogge, J.B. Building Lipid Barriers: Biosynthesis of Cutin and Suberin. Trends Plant Sci. 2008, 13, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Rizikovs, J.; Godina, D.; Makars, R.; Paze, A.; Abolins, A.; Fridrihsone, A.; Meile, K.; Kirpluks, M. Suberinic Acids as a Potential Feedstock for Polyol Synthesis: Separation and Characterization. Polymers 2021, 13, 4380. [Google Scholar] [CrossRef] [PubMed]
- Rizikovs, J.; Paze, A.; Makars, R.; Tupciauskas, R. A Method for Obtaining Thermoreactive Binders for a Production of Wood Composite Materials From Birch Outer Bark. 2021. Available online: https://patents.google.com/patent/EP3807341A1/ (accessed on 30 May 2022).
- Godiņa, D.; Paze, A.; Rizhikovs, J.; Stankus, K.; Virsis, I.; Nakurte, I. Stability Studies of Bioactive Compounds from Birch Outer Bark Ethanolic Extracts. Key Eng. Mater. 2018, 762, 152–157. [Google Scholar] [CrossRef]
- ISO 1171:2010; Solid Mineral Fuels—Determination of Ash. International Organization for Standardization: Geneva, Switzerland, 2010.
- EN 323:2000; Wood-Based Panels—Determination of Density. European Committee for Standardisation: Brussels, Belgium, 2000.
- EN 310:2001; Wood-Based Panels—Determination of Modulus of Elasticity in Bending and of Bending Strength. European Committee for Standardisation: Brussels, Belgium, 2001.
- EN 317:2000; Particleboards and Fibreboards—Determination of Swelling in Thickness after Immersion in Water. European Committee for Standardisation: Brussels, Belgium, 2000.
- EN 319:2000; Particleboards and Fibreboards—Determination of Tensile Strength Perpendicular to the Plane of the Board. European Committee for Standardisation: Brussels, Belgium, 2000.
- EN 312:2011; Particleboards—Specifications. European Committee for Standardisation: Brussels, Belgium, 2011.
- Sagnelli, D.; Vestri, A.; Curia, S.; Taresco, V.; Santagata, G.; Johansson, M.K.G.; Howdle, S.M. Green Enzymatic Synthesis and Processing of Poly (Cis-9,10-Epoxy-18-Hydroxyoctadecanoic Acid) in Supercritical Carbon Dioxide (ScCO2). Eur. Polym. J. 2021, 161, 110827. [Google Scholar] [CrossRef]
- Shangguan, W.; Chen, Z.; Zhao, J.; Song, X. Thermogravimetric Analysis of Cork and Cork Components from Quercus Variabilis. Wood Sci. Technol. 2018, 52, 181–192. [Google Scholar] [CrossRef]
- Şen, U.; Pereira, H. Pyrolysis Behavior of Alternative Cork Species. J. Therm. Anal. Calorim. 2022, 147, 4017–4025. [Google Scholar] [CrossRef]
- Shiqian, W.; Xiaozhou, S.; Yafang, L.; Mingqiang, Z. Characterizations and Properties of Torrefied Quercus Variabilis Cork. Wood Res. 2018, 63, 947–958. [Google Scholar]
- Rizhikovs, J.; Brazdausks, P.; Paze, A.; Tupciauskas, R.; Grinins, J.; Puke, M.; Plavniece, A.; Andzs, M.; Godina, D.; Makars, R. Characterization of Suberinic Acids from Birch Outer Bark as Bio-Based Adhesive in Wood Composites. Int. J. Adhes. Adhes. 2022, 112, 102989. [Google Scholar] [CrossRef]
- Graça, J.; Pereira, H. Methanolysis of Bark Suberins: Analysis of Glycerol and Acid Monomers. Phytochem. Anal. 2000, 11, 45–51. [Google Scholar] [CrossRef]
- Karnaouri, A.; Rova, U.; Christakopoulos, P. Effect of Different Pretreatment Methods on Birch Outer Bark: New Biorefinery Routes. Molecules 2016, 21, 427. [Google Scholar] [CrossRef] [PubMed]








| Solvent/Sample | KOH, g L−1 | BOB: Solvent, g L−1 | Temperature, °C |
|---|---|---|---|
| MeOH | 41.5 | 100 | 66 |
| EtOH 1 | 29.2 | 100 | 80 |
| i-PrOH | 41.5 | 100 | 80 |
| BuOH | 41.5 | 100 | 80 |
| Variable | Factor Level | |
|---|---|---|
| Low | High | |
| c, wt% | 20 | 40 |
| T, °C | 200 | 250 |
| t, min | 2 | 8 |
| Cycle | t = 2 min | t = 8 min |
|---|---|---|
| 1 | 3.5 MPa (1 min 20 s) | 3.5 MPa (2 min 30 s) |
| 0.1 MPa (10 s) | 0.1 MPa (30 s) | |
| 2 | 1.7 MPa (20 s) | 1.7 MPa (2 min) |
| 0.7 MPa (10 s) | 0.1 MPa (30 s) | |
| 3 | - | 1.7 MPa (2 min) |
| - | 0.7 MPa (30 s) |
| Sample | Acid Number, mg KOH g−1 | Epoxy Groups, mmol g−1 | Soluble Substance in THF, wt% | Ash Content, wt% |
|---|---|---|---|---|
| MeOH | 70.9 | 0.45 | 44.0 | 12.9 |
| EtOH | 95.8 | 0.61 | 51.8 | 6.7 |
| i-PrOH | 122.0 | 0.11 | 58.1 | 9.3 |
| BuOH | 91.1 | 0.25 | 57.5 | 6.6 |
| Sample | Mn, kDa | Mw, kDa | Mw/Mn |
|---|---|---|---|
| MeOH | 9.886 | 7526 | 761 |
| EtOH | 9.036 | 6577 | 728 |
| i-PrOH | 3.926 | 1600 | 407 |
| BuOH | 10.502 | 7525 | 717 |
| Sample | Cellulose, wt% | Aromatic Suberin + Lignin, wt% | ω-Hydroxy Acids, wt% | α, ω-Diacids, wt% |
|---|---|---|---|---|
| MeOH | 9.2 | 20.3 | 12.2 | 13.3 |
| EtOH | 9.0 | 21.4 | 17.5 | 11.9 |
| i-PrOH | 11.3 | 22.3 | 13.8 | 13.9 |
| BuOH | 8.3 | 22.1 | 14.2 | 11.2 |
| Adhesive_c | MOE, N mm−2 | MOR, N mm−2 | TS 24 h, % | Density, g cm−3 |
|---|---|---|---|---|
| MeOH_20 | 1690 | 6.18 | 32.1 | 0.847 |
| MeOH_30 | 1211 | 5.65 | 24.5 | 0.857 |
| MeOH_40 | 1572 | 5.19 | 19.0 | 0.884 |
| EtOH_20 | 2266 | 8.39 | 23.6 | 0.855 |
| EtOH_30 | 2331 | 7.79 | 14.7 | 0.867 |
| EtOH_40 | 2040 | 6.99 | 10.1 | 0.864 |
| i-PrOH_20 | 2866 | 9.55 | 16.1 | 0.860 |
| i-PrOH_30 | 2862 | 10.17 | 10.9 | 0.869 |
| i-PrOH_40 | 2459 | 8.23 | 8.0 | 0.864 |
| BuOH_20 | 2036 | 5.96 | 14.3 | 0.880 |
| BuOH_30 | 1934 | 6.26 | 11.7 | 0.868 |
| BuOH_40 | 1292 | 4.98 | 7.4 | 0.869 |
| EN 312 P3 1 | ≥2050 | ≥15 | ≤17 | – |
| EN 312 P2 2 | ≥1600 | ≥11 | – | – |
| Variable Parameters | MOE, N mm−2 | MOR, N mm−2 | TS 24 h, % | Density, g cm−3 | ||
|---|---|---|---|---|---|---|
| c, wt% | T, °C | t, min | ||||
| 20 | 210 | 2 | 659 | 2.08 | 41.8 | 0.688 |
| 20 | 210 | 8 | 1831 | 6.63 | 22.5 | 0.854 |
| 20 | 250 | 2 | 2820 | 7.64 | 17.3 | 0.845 |
| 20 | 250 | 8 | 3518 | 11.44 | 3.9 | 0.846 |
| 40 | 210 | 2 | 1403 | 5.45 | 20.9 | 0.844 |
| 40 | 210 | 8 | 2187 | 7.77 | 12.4 | 0.891 |
| 40 | 250 | 2 | 2612 | 7.92 | 8.3 | 0.880 |
| 40 | 250 | 8 | 2723 | 10.31 | 2.1 | 0.853 |
| Variable Parameters | MOE, N mm−2 | MOR, N mm−2 | TS 24 h, % | Density, g cm−3 | ||
|---|---|---|---|---|---|---|
| c, wt% | T, °C | t, min | ||||
| 29 | 232 | 5.63 | 2709 | 9.51 | 11.9 | 0.894 |
| 40 | 219 | 5.00 | 2149 | 7.07 | 12.3 | 0.876 |
| 29 | 224 | 5.90 | 2453 | 8.35 | 14.0 | 0.883 |
| 29 | 217 | 4.70 | 2073 | 7.34 | 18.6 | 0.863 |
| 33.5 | 230 | 2.00 | 1593 | 5.49 | 19.2 | 0.850 |
| 39 | 240 | 5.03 | 2679 | 8.74 | 6.9 | 0.896 |
| 20 | 233 | 5.30 | 2840 | 9.03 | 15.6 | 0.889 |
| 30 | 250 | 3.59 | 2951 | 8.76 | 7.0 | 0.870 |
| Value | Goal | Lower Limit | Upper Limit |
|---|---|---|---|
| c, wt% | is in range | 20 | 40 |
| T, °C | minimize | 210 | 250 |
| t, min | minimize | 2 | 8 |
| MOE N, mm−2 | maximize | 1800 | 3917 |
| MOR N, mm−2 | maximize | 11.00 | 12.59 |
| TS 24 h, % | minimize | 1.88 | 44.94 |
| Density, g cm−3 | is target = 0.83 | 0.671 | 0.952 |
| Response Value | Result | 95% Prediction Interval Lower Limit | 95% Prediction Interval Upper Limit |
|---|---|---|---|
| MOE, N mm−2 | 3833 | 3249 | 3939 |
| MOR, N mm−2 | 11.27 | 10.17 | 12.55 |
| TS 24 h, % | 6.26 | 4.49 | 7.12 |
| Density, g cm−3 | 0.903 | 0.837 | 0.913 |
| IB, N mm−2 | 1.33 1 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makars, R.; Rizikovs, J.; Godina, D.; Paze, A.; Merijs-Meri, R. Utilization of Suberinic Acids Containing Residue as an Adhesive for Particle Boards. Polymers 2022, 14, 2304. https://doi.org/10.3390/polym14112304
Makars R, Rizikovs J, Godina D, Paze A, Merijs-Meri R. Utilization of Suberinic Acids Containing Residue as an Adhesive for Particle Boards. Polymers. 2022; 14(11):2304. https://doi.org/10.3390/polym14112304
Chicago/Turabian StyleMakars, Raimonds, Janis Rizikovs, Daniela Godina, Aigars Paze, and Remo Merijs-Meri. 2022. "Utilization of Suberinic Acids Containing Residue as an Adhesive for Particle Boards" Polymers 14, no. 11: 2304. https://doi.org/10.3390/polym14112304
APA StyleMakars, R., Rizikovs, J., Godina, D., Paze, A., & Merijs-Meri, R. (2022). Utilization of Suberinic Acids Containing Residue as an Adhesive for Particle Boards. Polymers, 14(11), 2304. https://doi.org/10.3390/polym14112304