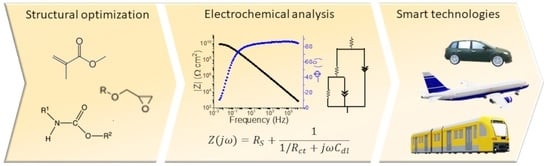
Electrochemical Characterization of Polymeric Coatings for Corrosion Protection: A Review of Advances and Perspectives
Abstract
:1. Introduction

2. Characterization of Polymeric Coatings
2.1. Accelerated Corrosion Tests
Salt-Spray Tests
2.2. Conventional Electrochemical Analyses
2.2.1. Open-Circuit Potential (OCP)
2.2.2. Electrochemical Impedance Spectroscopy (EIS)
2.3. Advanced Electrochemical Analyses
2.3.1. Localized Electrochemical Impedance Spectroscopy (LEIS)
2.3.2. Scanning Vibrating Electrode Technique (SVET)

2.3.3. Scanning Ion-Selective Electrode Technique (SIET)
2.3.4. Scanning Kelvin Probe (SKP)
2.3.5. Scanning Electrochemical Microscopy (SECM)
2.4. Theoretical Approaches
3. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harris, W. Corrosion, its Causes, Cost and Prevention: A Brief History of the Growth of Corrosion Study. Anti-Corros. Methods Mater. 1957, 4, 162–164. [Google Scholar] [CrossRef]
- Flournoy, R.W. Topic of the month—Preliminary Evaluation of Protective Coating Systems. Corrosion 1955, 11, 17–18. [Google Scholar] [CrossRef]
- Kendall, V.V.; Speller, F.N. Recent Developments in Corrosion Prevention of Ferrous Metals. Ind. Eng. Chem. 1931, 23, 735–742. [Google Scholar] [CrossRef]
- Orazem, M.E.; Tribollet, B. Electrochemical Impedance Spectroscopy; John Wiley & Sons: Hoboken, NJ, USA, 2008; Volume 48, ISBN 9780470381588. [Google Scholar]
- Wolstenholme, J. Electrochemical methods of assessing the corrosion of painted metals—A review. Corros. Sci. 1973, 13, 521–530. [Google Scholar] [CrossRef]
- Burns, R.M.; Haring, H.E. Determination of the Corrosion Behavior of Painted Iron and the Inhibitive Action of Paints. Bell Syst. Tech. J. 1936, 15, 343–348. [Google Scholar] [CrossRef]
- Fréchette, E.; Compere, C.; Ghali, E. Evaluation of the corrosion resistance of painted steels by impedance measurements. Corros. Sci. 1992, 33, 1067–1081. [Google Scholar] [CrossRef]
- Pourbaix, M. Applications of electrochemistry in corrosion science and in practice. Corros. Sci. 1974, 14, 25–82. [Google Scholar] [CrossRef]
- Wagner, C.; Traud, W. On the Interpretation of Corrosion Phenomena by Superposition of Electrochemical Partial Processes, and on the Potential of Mixed Electrodes. Z. Elektrochem. 1938, 44, 391–402. (In German) [Google Scholar]
- Allen, E.R., Jr. A Coating Evaluation Testing Program. Corrosion 1958, 14, 18–24. [Google Scholar] [CrossRef]
- Schneider, W.R.; Hendrickson, D. Electrical Measurements Applied to Corrosion Investigations. Corrosion 1954, 10, 337–342. [Google Scholar] [CrossRef]
- Bacon, R.C.; Smith, J.J.; Rugg, F.M. Electrolytic Resistance in Evaluating Protective Merit of Coatings on Metals. Ind. Eng. Chem. 1948, 40, 161–167. [Google Scholar] [CrossRef]
- Mayne, J. How paints prevent corrosion. Anti-Corros. Methods Mater. 1954, 1, 286–290. [Google Scholar] [CrossRef]
- Brown, J.R. Evaluation of Protective Coatings for Ship Bottoms. Corrosion 1959, 15, 49–54. [Google Scholar] [CrossRef]
- House, L.H. Instruments and Corrosion. Corros. Technol. 1960, 7, 222–223. [Google Scholar]
- Epelboin, I.; Keddam, M.; Takenouti, H. Use of impedance measurements for the determination of the instant rate of metal corrosion. J. Appl. Electrochem. 1972, 2, 71–79. [Google Scholar] [CrossRef]
- Loveday, D.; Peterson, P.; Rodgers, B. Evaluation of Organic Coatings with Electrochemical Impedance Spectroscopy. Part 2: Application of EIS to Coatings. J. Coat. Technol. 2004, 1, 88–93. [Google Scholar]
- Lillard, R.S.; Moran, P.J.; Isaacs, H.S. A Novel Method for Generating Quantitative Local Electrochemical Impedance Spectroscopy. J. Electrochem. Soc. 1992, 139, 1007–1012. [Google Scholar] [CrossRef]
- Rowlands, J.C.; Chuter, D.J. A.C. impedance measurements on marine paint systems. Corros. Sci. 1983, 23, 331–340. [Google Scholar] [CrossRef]
- Walter, G. A review of impedance plot methods used for corrosion performance analysis of painted metals. Corros. Sci. 1986, 26, 681–703. [Google Scholar] [CrossRef]
- Isaacs, H.S.; Kendig, M.W. Determination of Surface Inhomogeneities Using a Scanning Probe Impedance Technique. Corrosion 1980, 36, 269–274. [Google Scholar] [CrossRef]
- Frankel, G.S. Fundamentals of Corrosion Kinetics. In Active Protective Coatings: New-Generation Coatings for Metals; Springer: Dordrecht, The Netherlands, 2016; pp. 17–32. ISBN 978-94-017-7538-0. [Google Scholar]
- Alcántara, J.; De La Fuente, D.; Chico, B.; Simancas, J.; Díaz, I.; Morcillo, M. Marine Atmospheric Corrosion of Carbon Steel: A Review. Materials 2017, 10, 406. [Google Scholar] [CrossRef] [Green Version]
- Ma, F.-Y. Corrosive Effects of Chlorides on Metals. In Pitting Corrosion; Bensalah, N., Ed.; InTech: Rijeka, Croatia, 2012; pp. 139–178. ISBN 978-953-51-0275-5. [Google Scholar]
- Foley, R.T. Localized Corrosion of Aluminum Alloys—A Review. Corrosion 1986, 42, 277–288. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Foley, R.T. On the Mechanism of Pitting of Aluminum. J. Electrochem. Soc. 1979, 126, 1855–1860. [Google Scholar] [CrossRef]
- Grundmeier, G.; Schmidt, W.; Stratmann, M. Corrosion protection by organic coatings: Electrochemical mechanism and novel methods of investigation. Electrochim. Acta 2000, 45, 2515–2533. [Google Scholar] [CrossRef]
- Trentin, A.; Gasparini, A.D.L.; Faria, F.A.; Harb, S.V.; Dos Santos, F.C.; Pulcinelli, S.H.; Santilli, C.V.; Hammer, P. Barrier properties of high performance PMMA-silica anticorrosion coatings. Prog. Org. Coat. 2020, 138, 105398. [Google Scholar] [CrossRef]
- Harb, S.V.; Trentin, A.; Torrico, R.F.O.; Pulcinelli, S.H.; Santilli, C.V.; Hammer, P. Organic-Inorganic Hybrid Coatings for Corrosion Protection of Metallic Surfaces. In New Technologies in Protective Coatings; Giudice, C., Canosa, G., Eds.; InTech: Rijeka, Croatia, 2017. [Google Scholar]
- Echeverría, M.; Abreu, C.; Lau, K.; Echeverría, C. Viability of epoxy–siloxane hybrid coatings for preventing steel corrosion. Prog. Org. Coat. 2016, 92, 29–43. [Google Scholar] [CrossRef]
- Zadeh, M.A.; Van der Zwaag, S.; Garcia, S.J. Adhesion and Long-Term Barrier Restoration of Intrinsic Self-Healing Hybrid Sol–Gel Coatings. ACS Appl. Mater. Interfaces 2016, 8, 4126–4136. [Google Scholar] [CrossRef]
- Mosa, J.; Rosero-Navarro, N.C.; Aparicio, M. Active corrosion inhibition of mild steel by environmentally-friendly Ce-doped organic–inorganic sol–gel coatings. RSC Adv. 2016, 6, 39577–39586. [Google Scholar] [CrossRef]
- Trentin, A.; Harb, S.V.; Uvida, M.C.; Pulcinelli, S.H.; Santilli, C.V.; Marcoen, K.; Pletincx, S.; Terryn, H.; Hauffman, T.; Hammer, P. Dual Role of Lithium on the Structure and Self-Healing Ability of PMMA-Silica Coatings on AA7075 Alloy. ACS Appl. Mater. Interfaces 2019, 11, 40629–40641. [Google Scholar] [CrossRef]
- Habib, S.; Fayyed, E.; Shakoor, R.A.; Kahraman, R.; Abdullah, A. Improved self-healing performance of polymeric nanocomposites reinforced with talc nanoparticles (TNPs) and urea-formaldehyde microcapsules (UFMCs). Arab. J. Chem. 2021, 14, 102926. [Google Scholar] [CrossRef]
- Liu, Y.; Visser, P.; Zhou, X.; Lyon, S.B.; Hashimoto, T.; Curioni, M.; Gholinia, A.; Thompson, G.E.; Smyth, G.; Gibbon, S.R.; et al. Protective Film Formation on AA2024-T3 Aluminum Alloy by Leaching of Lithium Carbonate from an Organic Coating. J. Electrochem. Soc. 2016, 163, C45. [Google Scholar] [CrossRef] [Green Version]
- Blaiszik, B.; Kramer, S.; Olugebefola, S.; Moore, J.; Sottos, N.; White, S. Self-Healing Polymers and Composites. Annu. Rev. Mater. Sci. 2010, 40, 179–211. [Google Scholar] [CrossRef]
- White, S.R.; Sottos, N.R.; Geubelle, P.H.; Moore, J.S.; Kessler, M.R.; Sriram, S.R.; Brown, E.N.; Viswanathan, S. Autonomic healing of polymer composites. Nature 2001, 409, 794–797. [Google Scholar] [CrossRef]
- Baghdachi, J. Smart Coatings. In Smart Coatings II; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2009; Volume 1002, pp. 3–24. ISBN 9780841272187. [Google Scholar]
- Trentin, A.; Uvida, M.C.; De Araújo Almeida, A.; De Souza, T.A.C.; Hammer, P. Self-Healing Nanocoatings. In Nanotechnology in the Automotive Industry; Song, H., Nguyen, T.A., Yasin, G., Singh, N.B., Gupta, R.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 371–401. ISBN 9788490225370. [Google Scholar]
- Jin, Z.; Yang, L.; Shi, S.; Wang, T.; Duan, G.; Liu, X.; Li, Y. Flexible Polydopamine Bioelectronics. Adv. Funct. Mater. 2021, 31, 2103391. [Google Scholar] [CrossRef]
- Wang, L.; Deng, L.; Zhang, D.; Qian, H.; Du, C.; Li, X.; Mol, A.; Terryn, H. Shape memory composite (SMC) self-healing coatings for corrosion protection. Prog. Org. Coat. 2016, 97, 261–268. [Google Scholar] [CrossRef]
- Yang, P.; Zhu, F.; Zhang, Z.; Cheng, Y.; Wang, Z.; Li, Y. Stimuli-responsive polydopamine-based smart materials. Chem. Soc. Rev. 2021, 50, 8319–8343. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, C.; Li, L.; Zhu, F.; Ren, X.; Huang, Q.; Cheng, Y.; Li, Y. Bioinspired Integration of Naturally Occurring Molecules towards Universal and Smart Antibacterial Coatings. Adv. Funct. Mater. 2022, 32, 2108749. [Google Scholar] [CrossRef]
- Cho, S.H.; White, S.R.; Braun, P.V. Self-Healing Polymer Coatings. Adv. Mater. 2009, 21, 645–649. [Google Scholar] [CrossRef]
- Dieleman, C.D.; Denissen, P.J.; Garcia, S.J. Long-Term Active Corrosion Protection of Damaged Coated-AA2024-T3 by Embedded Electrospun Inhibiting Nanonetworks. Adv. Mater. Interfaces 2018, 5, 1800176. [Google Scholar] [CrossRef]
- Fandi, M.; Liu, L. Electrochemical Evaluation Technologies of Organic Coatings. In Coatings and Thin-Film Technologies; Jaime, A.P.-T., Bernal, A.G.A., Eds.; IntechOpen: London, UK, 2018; pp. 49–67. ISBN 978-1-78984-871-7. [Google Scholar]
- Xia, D.-H.; Deng, C.-M.; Macdonald, D.; Jamali, S.; Mills, D.; Luo, J.-L.; Strebl, M.G.; Amiri, M.; Jin, W.; Song, S.; et al. Electrochemical measurements used for assessment of corrosion and protection of metallic materials in the field: A critical review. J. Mater. Sci. Technol. 2022, 112, 151–183. [Google Scholar] [CrossRef]
- Meade, C.L. Accelerated corrosion testing. Met. Finish. 1999, 97, 526–531. [Google Scholar] [CrossRef]
- Rohwerder, M. Conducting polymers for corrosion protection: A review. Int. J. Mater. Res. 2009, 10, 1331–1342. [Google Scholar] [CrossRef]
- Deshpande, P.P.; Jadhav, N.G.; Gelling, V.J.; Sazou, D. Conducting polymers for corrosion protection: A review. J. Coat. Technol. Res. 2014, 11, 473–494. [Google Scholar] [CrossRef]
- Zadeh, M.K.; Yeganeh, M.; Shoushtari, M.T.; Esmaeilkhanian, A. Corrosion performance of polypyrrole-coated metals: A review of perspectives and recent advances. Synth. Met. 2021, 274, 116723. [Google Scholar] [CrossRef]
- Ates, M. A review on conducting polymer coatings for corrosion protection. J. Adhes. Sci. Technol. 2016, 30, 1510–1536. [Google Scholar] [CrossRef]
- Namsheer, K.; Rout, C.S. Conducting polymers: A comprehensive review on recent advances in synthesis, properties and applications. RSC Adv. 2021, 11, 5659–5697. [Google Scholar] [CrossRef]
- Holden, H. How carefully do you control your salt spray test? Anti-Corros. Methods Mater. 1955, 2, 157–163. [Google Scholar] [CrossRef]
- Bierwagen, G.P.; He, L.; Li, J.; Ellingson, L.; Tallman, D. Studies of a new accelerated evaluation method for coating corrosion resistance—Thermal cycling testing. Prog. Org. Coat. 2000, 39, 67–78. [Google Scholar] [CrossRef]
- Blakey, R. Evaluation of paint durability-natural and accelerated. Prog. Org. Coat. 1985, 13, 279–296. [Google Scholar] [CrossRef]
- ASTM B117-16; Standard Practice for Operating Salt Spray (Fog) Apparatus. ASTM International: West Conehohocken, PA, USA, 2016.
- Lazzari, L. Testing. In Engineering Tools for Corrosion; Lazzari, L., Ed.; Woodhead Publishing: Cambridge, UK, 2017; pp. 119–129. ISBN 9780081024249. [Google Scholar]
- Visser, P.; Liu, Y.; Terryn, H.; Mol, J.M.C. Lithium salts as leachable corrosion inhibitors and potential replacement for hexavalent chromium in organic coatings for the protection of aluminum alloys. J. Coat. Technol. Res. 2016, 13, 557–566. [Google Scholar] [CrossRef] [Green Version]
- Su, Y.; Qiu, S.; Liu, Y.; Yang, D.; Zhao, H.; Wang, L. PEDOT: PSS-exfoliated Graphene to Improve the Corrosion Resistance of Waterborne Epoxy Coating. Int. J. Electrochem. Sci. 2019, 14, 4595–4610. [Google Scholar] [CrossRef]
- Varshney, S.; Chugh, K.; Mhaske, S.T. Effect of layer-by-layer synthesized graphene–polyaniline-based nanocontainers for corrosion protection of mild steel. J. Mater. Sci. 2022, 57, 8348–8366. [Google Scholar] [CrossRef]
- Lei, Y.; Qiu, Z.; Tan, N.; Du, H.; Li, D.; Liu, J.; Liu, T.; Zhang, W.; Chang, X. Polyaniline/CeO2 nanocomposites as corrosion inhibitors for improving the corrosive performance of epoxy coating on carbon steel in 3.5% NaCl solution. Prog. Org. Coat. 2020, 139, 105430. [Google Scholar] [CrossRef]
- Thuy, D.N.; Xuan, H.T.T.; Nicolay, A.; Paint, Y.; Olivier, M.-G. Corrosion protection of carbon steel by solvent free epoxy coating containing hydrotalcites intercalated with different organic corrosion inhibitors. Prog. Org. Coat. 2016, 101, 331–341. [Google Scholar] [CrossRef]
- Castro, Y.; Özmen, E.; Durán, A. Integrated self-healing coating system for outstanding corrosion protection of AA2024. Surf. Coat. Technol. 2020, 387, 125521. [Google Scholar] [CrossRef]
- Zheludkevich, M.; Poznyak, S.; Rodrigues, L.; Raps, D.; Hack, T.; Dick, L.F.P.; Nunes, T.; Ferreira, M.G.S. Active protection coatings with layered double hydroxide nanocontainers of corrosion inhibitor. Corros. Sci. 2010, 52, 602–611. [Google Scholar] [CrossRef]
- Díaz, I.; Chico, B.; De la Fuente, D.; Simancas, J.; Vega, J.; Morcillo, M. Corrosion resistance of new epoxy–siloxane hybrid coatings. A laboratory study. Prog. Org. Coat. 2010, 69, 278–286. [Google Scholar] [CrossRef] [Green Version]
- Boumezgane, O.; Suriano, R.; Fedel, M.; Tonelli, C.; Deflorian, F.; Turri, S. Self-healing epoxy coatings with microencapsulated ionic PDMS oligomers for corrosion protection based on supramolecular acid-base interactions. Prog. Org. Coat. 2022, 162, 106558. [Google Scholar] [CrossRef]
- Ohtsuka, T. Corrosion Protection of Steels by Conducting Polymer Coating. Int. J. Corros. 2012, 2012, 915090. [Google Scholar] [CrossRef]
- Bonora, P.; Deflorian, F.; Fedrizzi, L. Electrochemical impedance spectroscopy as a tool for investigating underpaint corrosion. Electrochim. Acta 1996, 41, 1073–1082. [Google Scholar] [CrossRef]
- Razin, A.A.; Ramezanzadeh, B.; Yari, H. Detecting and estimating the extent of automotive coating delamination and damage indexes after stone chipping using electrochemical impedance spectroscopy. Prog. Org. Coat. 2016, 92, 95–109. [Google Scholar] [CrossRef]
- Gonzalez-Garcia, Y.; Espallargas, S.G.; Mol, J.M.C. Electrochemical Techniques for the Study of Self Healing Coatings. In Active Protective Coatings: New-Generation Coatings for Metals; Hughes, A.E., Mol, J.M.C., Zheludkevich, M.L., Buchheit, R.G., Eds.; Springer: Dordrecht, The Netherlands, 2016; pp. 203–240. ISBN 9783642275111. [Google Scholar]
- Ammar, S.; Ramesh, K.; Ma, I.; Farah, Z.; Vengadaesvaran, B.; Ramesh, S.; Arof, A.K. Studies on SiO2-hybrid polymeric nanocomposite coatings with superior corrosion protection and hydrophobicity. Surf. Coat. Technol. 2017, 324, 536–545. [Google Scholar] [CrossRef]
- Bouvet, G.; Nguyen, D.D.; Mallarino, S.; Touzain, S. Analysis of the non-ideal capacitive behaviour for high impedance organic coatings. Prog. Org. Coat. 2014, 77, 2045–2053. [Google Scholar] [CrossRef]
- Nguyen, A.S.; Musiani, M.; Orazem, M.E.; Pébère, N.; Tribollet, B.; Vivier, V. Impedance analysis of the distributed resistivity of coatings in dry and wet conditions. Electrochim. Acta 2015, 179, 452–459. [Google Scholar] [CrossRef] [Green Version]
- Snihirova, D.; Lamaka, S.; Montemor, F. “SMART” protective ability of water based epoxy coatings loaded with CaCO3 microbeads impregnated with corrosion inhibitors applied on AA2024 substrates. Electrochim. Acta 2012, 83, 439–447. [Google Scholar] [CrossRef]
- Vlasak, R.; Klueppel, I.; Grundmeier, G. Combined EIS and FTIR–ATR study of water uptake and diffusion in polymer films on semiconducting electrodes. Electrochim. Acta 2007, 52, 8075–8080. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Niroumandrad, S.; Ahmadi, A.; Mahdavian-Ahadi, M.; Moghadam, M.M. Enhancement of barrier and corrosion protection performance of an epoxy coating through wet transfer of amino functionalized graphene oxide. Corros. Sci. 2016, 103, 283–304. [Google Scholar] [CrossRef]
- Huang, V.M.; Wu, S.-L.; Orazem, M.E.; Pébère, N.; Tribollet, B.; Vivier, V. Local electrochemical impedance spectroscopy: A review and some recent developments. Electrochim. Acta 2011, 56, 8048–8057. [Google Scholar] [CrossRef] [Green Version]
- Calado, L.M.; Taryba, M.G.; Carmezim, M.J.; Montemor, M.F. Self-healing ceria-modified coating for corrosion protection of AZ31 magnesium alloy. Corros. Sci. 2018, 142, 12–21. [Google Scholar] [CrossRef]
- Kartsonakis, I.; Athanasopoulou, E.; Snihirova, D.; Martins, B.; Koklioti, M.; Montemor, M.; Kordas, G.; Charitidis, C. Multifunctional epoxy coatings combining a mixture of traps and inhibitor loaded nanocontainers for corrosion protection of AA2024-T3. Corros. Sci. 2014, 85, 147–159. [Google Scholar] [CrossRef]
- Jadhav, N.; Gelling, V.J. Review—The Use of Localized Electrochemical Techniques for Corrosion Studies. J. Electrochem. Soc. 2019, 166, C3461–C3476. [Google Scholar] [CrossRef]
- Lutz, A.; Van den Berg, O.; Wielant, J.; De Graeve, I.; Terryn, H. A Multiple-Action Self-Healing Coating. Front. Mater. 2016, 2, 73. [Google Scholar] [CrossRef] [Green Version]
- Calado, L.M.; Taryba, M.G.; Morozov, Y.; Carmezim, M.; Montemor, M.F. Novel smart and self-healing cerium phosphate-based corrosion inhibitor for AZ31 magnesium alloy. Corros. Sci. 2020, 170, 108648. [Google Scholar] [CrossRef]
- Yasakau, K.; Carneiro, J.; Zheludkevich, M.; Ferreira, M. Influence of sol-gel process parameters on the protection properties of sol–gel coatings applied on AA2024. Surf. Coat. Technol. 2014, 246, 6–16. [Google Scholar] [CrossRef]
- Lamaka, S.V.; Souto, R.M.; Ferreira, M.G.S. In-Situ Visualization of Local Corrosion by Scanning Ion-Selective Electrode Technique (SIET). In Microscopy: Science, Technology, Applications and Education; Formatex Research Center: Badajoz, Spain, 2010; Volume 3, pp. 2162–2173. [Google Scholar]
- Yasakau, K.A.; Zheludkevich, M.L.; Lamaka, S.V.; Ferreira, M.G.S. Mechanism of Corrosion Inhibition of AA2024 by Rare-Earth Compounds. J. Phys. Chem. B 2006, 110, 5515–5528. [Google Scholar] [CrossRef]
- Visser, P.; Lutz, A.; Mol, J.; Terryn, H. Study of the formation of a protective layer in a defect from lithium-leaching organic coatings. Prog. Org. Coat. 2016, 99, 80–90. [Google Scholar] [CrossRef]
- Córdoba, L.C.; Marques, A.; Taryba, M.; Coradin, T.; Montemor, F. Hybrid coatings with collagen and chitosan for improved bioactivity of Mg alloys. Surf. Coat. Technol. 2018, 341, 103–113. [Google Scholar] [CrossRef]
- Örnek, C.; Leygraf, C.; Pan, J. On the Volta potential measured by SKPFM–fundamental and practical aspects with relevance to corrosion science. Corros. Eng. Sci. Technol. 2019, 54, 185–198. [Google Scholar] [CrossRef] [Green Version]
- Bai, X.; Tran, T.H.; Yu, D.; Vimalanandan, A.; Hu, X.; Rohwerder, M. Novel conducting polymer based composite coatings for corrosion protection of zinc. Corros. Sci. 2015, 95, 110–116. [Google Scholar] [CrossRef]
- Yin, Y.; Prabhakar, M.; Ebbinghaus, P.; Da Silva, C.C.; Rohwerder, M. Neutral inhibitor molecules entrapped into polypyrrole network for corrosion protection. Chem. Eng. J. 2022, 440, 135739. [Google Scholar] [CrossRef]
- Yin, Y.; Schulz, M.; Rohwerder, M. Optimizing smart self-healing coatings: Investigating the transport of active agents from the coating towards the defect. Corros. Sci. 2021, 190, 109661. [Google Scholar] [CrossRef]
- Klimow, G.; Fink, N.; Grundmeier, G. Electrochemical studies of the inhibition of the cathodic delamination of organically coated galvanised steel by thin conversion films. Electrochim. Acta 2007, 53, 1290–1299. [Google Scholar] [CrossRef]
- Posner, R.; Giza, G.; Vlasak, R.; Grundmeier, G. In situ electrochemical Scanning Kelvin Probe Blister-Test studies of the de-adhesion kinetics at polymer/zinc oxide/zinc interfaces. Electrochim. Acta 2009, 54, 4837–4843. [Google Scholar] [CrossRef]
- Posner, R.; Wapner, K.; Amthor, S.; Roschmann, K.; Grundmeier, G. Electrochemical investigation of the coating/substrate interface stability for styrene/acrylate copolymer films applied on iron. Corros. Sci. 2010, 52, 37–44. [Google Scholar] [CrossRef]
- Nazarov, A.P.; Thierry, D. Mechanism of the corrosion exfoliation of a polymer coating from a carbon steel. Prot. Met. Phys. Chem. Surfaces 2009, 45, 735–745. [Google Scholar] [CrossRef]
- Wu, J.; Peng, D.; Junsheng, W.; Du, X.; Zhang, Z.; Zhang, B.; Li, X.; Huang, Y. In Situ Formation of Decavanadate-Intercalated Layered Double Hydroxide Films on AA2024 and their Anti-Corrosive Properties when Combined with Hybrid Sol Gel Films. Materials 2017, 10, 426. [Google Scholar] [CrossRef] [Green Version]
- Lorenz, W.; Mansfeld, F. Determination of corrosion rates by electrochemical DC and AC methods. Corros. Sci. 1981, 21, 647–672. [Google Scholar] [CrossRef]
- Mansfeld, F.; Lin, S.; Kim, S.; Shih, H. Electrochemical impedance spectroscopy as a monitoring tool for passivation and localized Corrosion of aluminum alloys. Mater. Corros. 1988, 39, 487–492. [Google Scholar] [CrossRef]
- Dwivedi, D.; Lepková, K.; Becker, T. Carbon steel corrosion: A review of key surface properties and characterization methods. RSC Adv. 2017, 7, 4580–4610. [Google Scholar] [CrossRef] [Green Version]
- Hinderliter, B.R.; Croll, S.G.; Tallman, D.E.; Su, Q.; Bierwagen, G.P. Interpretation of EIS data from accelerated exposure of coated metals based on modeling of coating physical properties. Electrochim. Acta 2006, 51, 4505–4515. [Google Scholar] [CrossRef]
- Musiani, M.; Orazem, M.; Pébère, N.; Tribollet, B.; Vivier, V. Determination of resistivity profiles in anti-corrosion coatings from constant-phase-element parameters. Prog. Org. Coat. 2014, 77, 2076–2083. [Google Scholar] [CrossRef]
- Chen, Y.-M.; Nguyen, A.S.; Orazem, M.E.; Tribollet, B.; Pébère, N.; Musiani, M.; Vivier, V. Identification of Resistivity Distributions in Dielectric Layers by Measurement Model Analysis of Impedance Spectroscopy. Electrochim. Acta 2016, 219, 312–320. [Google Scholar] [CrossRef] [Green Version]
- Bonin, P.; Roggero, A.; Caussé, N.; Pébère, N.; Thierry, D.; Le Bozec, N. Impedance analysis of the barrier effect of coil-coated materials: Water uptake and glass transition variations. Prog. Org. Coat. 2021, 153, 106163. [Google Scholar] [CrossRef]
- Amand, S.; Musiani, M.; Orazem, M.; Pébère, N.; Tribollet, B.; Vivier, V. Constant-phase-element behavior caused by inhomogeneous water uptake in anti-corrosion coatings. Electrochim. Acta 2013, 87, 693–700. [Google Scholar] [CrossRef] [Green Version]
- Young, L. Anodic oxide films. Part 4—The interpretation of impedance measurements on oxide coated electrodes on niobium. Trans. Faraday Soc. 1955, 51, 1250–1260. [Google Scholar] [CrossRef]
- Brasher, D.M.; Kingsbury, A.H. Electrical measurements in the study of immersed paint coatings on metal. I. Comparison between capacitance and gravimetric methods of estimating water-uptake. J. Appl. Chem. 1954, 4, 62–72. [Google Scholar] [CrossRef]













| Technique | Information Provided | Advantages | Limitations | Ref. |
|---|---|---|---|---|
| Open circuit potential |
|
|
| [5,6,7,46] |
| EIS |
|
|
| [20,98,99] |
| LEIS |
|
|
| [27,71,81] |
| SVET |
|
|
| [27,71,78,81] |
| SIET |
|
|
| [81,85] |
| SKP |
|
|
| [27,81,89,100] |
| SECM |
|
|
| [71,81] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trentin, A.; Pakseresht, A.; Duran, A.; Castro, Y.; Galusek, D. Electrochemical Characterization of Polymeric Coatings for Corrosion Protection: A Review of Advances and Perspectives. Polymers 2022, 14, 2306. https://doi.org/10.3390/polym14122306
Trentin A, Pakseresht A, Duran A, Castro Y, Galusek D. Electrochemical Characterization of Polymeric Coatings for Corrosion Protection: A Review of Advances and Perspectives. Polymers. 2022; 14(12):2306. https://doi.org/10.3390/polym14122306
Chicago/Turabian StyleTrentin, Andressa, Amirhossein Pakseresht, Alicia Duran, Yolanda Castro, and Dušan Galusek. 2022. "Electrochemical Characterization of Polymeric Coatings for Corrosion Protection: A Review of Advances and Perspectives" Polymers 14, no. 12: 2306. https://doi.org/10.3390/polym14122306
APA StyleTrentin, A., Pakseresht, A., Duran, A., Castro, Y., & Galusek, D. (2022). Electrochemical Characterization of Polymeric Coatings for Corrosion Protection: A Review of Advances and Perspectives. Polymers, 14(12), 2306. https://doi.org/10.3390/polym14122306