Polymers Based on PLA from Synthesis Using D,L-Lactic Acid (or Racemic Lactide) and Some Biomedical Applications: A Short Review
Abstract
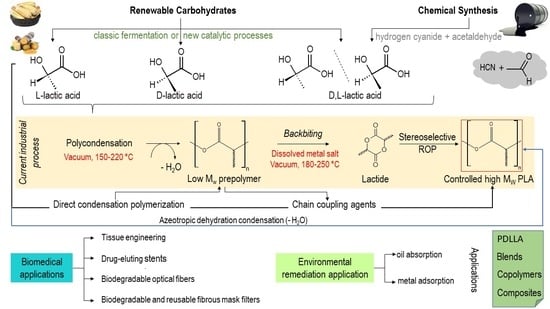
:1. Introduction
2. A Few Points about the Problem of Plastics
3. Monomers of Lactic Acid and Lactides to Produce PLA
4. Poly(lactic acid) (PLA) Polymers
4.1. A Brief Outline
4.2. Synthetic Routes
4.3. Structural Variety and PLA Properties
4.4. PLA Modifications: Blends, Copolymers and Composites
5. Materials of PLA Produced from D,L-Lactic Acid and Their Applications
5.1. Synthesis of PDLLA Using Different Catalysts
5.2. Synthesis of Blends, Copolymers and Composites Using PDLLA
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BnOH | benzyl alcohol |
| BTE | bone tissue engineering |
| CA | cholic acid |
| CgPCs | chitosan-graft-PDLLA copolymers |
| CIP | ciprofloxacin |
| CNCs | cellulose nanocrystals |
| CT | computerized tomography |
| CV | cresyl violet |
| D,L-LA | D,L-lactic acid |
| DBU | SQ-2 to SQ-6 and 1,8-diazabicyclo[5.4.0]undec-7-ene |
| DCM | dichloromethane |
| DESs | Drug-eluting stents |
| DM | Diabetes mellitus |
| DMA | Dimethylacetamide |
| DMAP | 4-dimethylaminopyridine |
| DMC | dimethyl carbonate |
| DMF | dimethylformamide |
| DMSO | dimethyl sulfoxide |
| DP-PDLLA | degree of polymerization of D,L-lactic acid |
| DSC | Differential Scanning Calorimetry |
| DS-PDLLA | degree of substitution of PDLLA |
| DTG | Derivative thermogravimetry |
| DTX | Docetaxel |
| EDC/NHS | N-hydroxysuccinimide/N-(3-dimethylaminopropyl)-N′-ethyl-carbodiimide hydrochloride |
| EDOT | 3,4-ethylenedioxythiphene |
| EG | ethylene glycol |
| El | elastin peptide |
| EmimAc | 1-ethyl-3-methyimidazolium acetate |
| ERY | erythromycin |
| ESC | enantiomorphic site control |
| FDM | fused deposition modeling |
| FT-IR | Fourier-transform infrared spectroscopy |
| GA | glycolic acid |
| GE | Gelatin |
| GMA-g-PEO | glycidyl methacrylate-g-poly(ethylene oxide) |
| GPC | Gel Permeation Chromatography |
| H3PW | 12-tungstofosforic acid |
| hc-PLA | PLA homocrystals |
| Hedta | ethylenediaminetetraacetic acid |
| HEMA | 2-hydroxyethyl methacrylate |
| HMW | High molecular weight |
| IONPs | iron oxide nanoparticles |
| LA | lactic acid |
| LAB | lactic acid bacteria |
| MBL | a-methylene-g-butyrolactone |
| m-LA | meso-lactide |
| Mn | number-average molecular weight |
| MN | microneedles |
| MP | microparticles |
| MPEG-PCLA | monomethoxy-poly(ethylene glycol)-poly(ε-caprolactone-co-D,L-lactide) |
| MRI | magnetic resonance imaging |
| MS | microspheres |
| MSA | methanesulfonic acid |
| MS-PDLLA | molar substitution of PDLLA |
| Mw | weight-average molecular weight |
| NEt3 | triethylamine |
| NNO | diaminophenoxy |
| NO | nitric oxide |
| NP | nanoparticles |
| OLA | oligo lactic acid |
| PB | prussian blue |
| PBDE | polybrominated diphenyl ether |
| PBNPs | prussian blue nanoparticles |
| PCL | poly(ε-caprolactone) |
| PDLA | poly(D-lactic acid) |
| PDLLA | poly(D,L-lactic acid) |
| PDLLA-b-PNVP | poly(D,L-lactide)-b-poly(N-vinylpyrrolidone) |
| PDP | phenyl dichlorophosphate |
| PEDOT | poly(3,4-ethylenedioxythiphene) |
| PEG | poly(ethylene glycol) |
| PEO | polyethylene oxide |
| PET | polyethylene terephthalate |
| PGPs | calcium phosphate-based glasses particulates |
| PHB | polyhydroxybutyrate |
| PLA | poly(lactic acid) |
| PLA/TPS | PLA-thermoplastic starch |
| PLA-g-MA | PLA-g-maleic anhydride |
| PLA-g-TPS | PLA-g-thermoplastic starch |
| PLEL | (PDLLA-PEG-PDLLA) hydrogel |
| PLGA | poly(lactic acid-co-glycolic acid) |
| PLLA | poly(L-lactic acid) |
| Pm | meso dyads |
| PNCs | polymer nanocomposites |
| PNVP | poly(N-vinylpyrrolidone) |
| PS | polystyrene |
| PTMC | poly(trimethylene carbonate) |
| PVA | poly(vinyl alcohol) |
| PVPh | poly(vinylphenol) |
| rac-LA | racemic lactide |
| RAFT | reversible addition-fragmentation chain transfer |
| Rapa | Rapamycin |
| ROP | Ring opening polymerization |
| sb-PLA | PLA stereoblocks |
| sc-PLA | PLA stereocomplexes |
| SEM | scanning electron microscopy |
| SnCl2 | tin(II) chloride dihydrate |
| SSF | solid-state foaming |
| SSP | solid-state polycondensation |
| STVPh | styrene-co-vinyl phenol |
| TBAC | tetrabutylammonium chloride |
| TBD | 1,5,7-triazabicyclo [4.4.0] dec-5-ene |
| Tg | glass transition temperature |
| TGA | thermogravimetric analysis |
| THF | tetrahydrofuran |
| Tm | melting temperature |
| TSA | p-toluenesulfonic acid |
| TUC’s | thiourea-based organocatalysts |
| WPDLLA | weight content of PDLLA |
| wt.% | weight percent |
| XRD | X-ray diffraction |
References
- Andrady, A.L.; Neal, M.A. Applications and societal benefits of plastics. Philos. Trans. R. Soc. B Biol. Sci. 2009, 36, 1977–1984. [Google Scholar] [CrossRef] [PubMed]
- Geyer, R. A Brief History of Plastics. In Mare Plasticum—The Plastic Sea; Springer International Publishing: Cham, Switzerland, 2020; pp. 31–47. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [Green Version]
- Blettler, M.C.M.; Wantzen, K.M. Threats Underestimated in Freshwater Plastic Pollution: Mini-Review. Water Air Soil Pollut. 2019, 230, 174. [Google Scholar] [CrossRef]
- Hale, R.C.; Seeley, M.E.; la Guardia, M.J.; Mai, L.; Zeng, E.Y. A Global Perspective on Microplastics. J. Geophys. Res. Ocean. 2020, 125, e2018JC01471. [Google Scholar] [CrossRef]
- Monteiro, R.C.P.; do Sul, J.A.I.; Costa, M.F. Plastic pollution in islands of the Atlantic Ocean. Environ. Pollut. 2018, 238, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.H.; He, Y.; Sen, B. Research and management of plastic pollution in coastal environments of China. Environ. Pollut. 2019, 248, 898–905. [Google Scholar] [CrossRef] [PubMed]
- Compa, M.; Alomar, C.; Wilcox, C.; van Sebille, E.; Lebreton, L.; Hardesty, B.D.; Deudero, S. Risk assessment of plastic pollution on marine diversity in the Mediterranean Sea. Sci. Total Environ. 2019, 678, 188–196. [Google Scholar] [CrossRef] [Green Version]
- Castro-Jiménez, J.; González-Fernández, D.; Fornier, M.; Schmidt, N.; Sempéré, R. Macro-litter in surface waters from the Rhone River: Plastic pollution and loading to the NW Mediterranean Sea. Mar. Pollut. Bull. 2019, 146, 60–66. [Google Scholar] [CrossRef]
- Tessnow-von Wysocki, I.; le Billon, P. Plastics at sea: Treaty design for a global solution to marine plastic pollution. Environ. Sci. Policy 2019, 100, 94–104. [Google Scholar] [CrossRef]
- Xu, L.; Cao, L.; Huang, W.; Liu, J.; Dou, S. Assessment of plastic pollution in the Bohai Sea: Abundance, distribution, morphological characteristics and chemical components. Environ. Pollut. 2021, 278, 116874. [Google Scholar] [CrossRef]
- Kosuth, M.; Mason, S.A.; Wattenberg, E.V. Anthropogenic contamination of tap water, beer, and sea salt. PLoS ONE 2018, 13, e0194970. [Google Scholar] [CrossRef]
- Yang, D.; Shi, H.; Li, L.; Li, J.; Jabeen, K.; Kolandhasamy, P. Microplastic Pollution in Table Salts from China. Environ. Sci. Technol. 2015, 49, 13622–13627. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Huang, W.; Chen, M.; Song, B.; Zeng, G.; Zhang, Y. (Micro)plastic crisis: Un-ignorable contribution to global greenhouse gas emissions and climate change. J. Clean. Prod. 2020, 254, 120138. [Google Scholar] [CrossRef]
- Prata, J.C. Airborne microplastics: Consequences to human health? Environ. Pollut. 2018, 234, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Song, B.; Zeng, G.; Zhang, Y.; Huang, W.; Wen, X.; Tang, W. Are biodegradable plastics a promising solution to solve the global plastic pollution? Environ. Pollut. 2020, 263, 114469. [Google Scholar] [CrossRef]
- Chen, X.; Yan, N. A brief overview of renewable plastics. Mater. Today Sustain. 2020, 7–8, 100031. [Google Scholar] [CrossRef]
- Andrady, A.L.; Rajapakse, N. Additives and Chemicals in Plastics. In The Handbook of Environmental Chemistry; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1–17. [Google Scholar] [CrossRef]
- Njembele, A.N.E.; Tremblay, J.J. Mechanisms of MEHP Inhibitory Action and Analysis of Potential Replacement Plasticizers on Leydig Cell Steroidogenesis. Int. J. Mol. Sci. 2021, 22, 11456. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P. Role of Plastics on Human Health. Indian J. Pediatr. 2018, 85, 384–389. [Google Scholar] [CrossRef]
- Burgos-Aceves, M.A.; Abo-Al-Ela, H.G.; Faggio, C. Physiological and metabolic approach of plastic additive effects: Immune cells responses. J. Hazard. Mater. 2021, 404, 124114. [Google Scholar] [CrossRef]
- Bi, X.; Pan, X.; Yuan, S.; Wang, Q. Plasticizer Contamination in Edible Vegetable Oil in a U.S. Retail Market. J. Agric. Food Chem. 2013, 61, 9502–9509. [Google Scholar] [CrossRef] [PubMed]
- Amiridou, D.; Voutsa, D. Alkylphenols and phthalates in bottled waters. J. Hazard. Mater. 2011, 185, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Mülhaupt, R. Green Polymer Chemistry and Bio-based Plastics: Dreams and Reality. Macromol. Chem. Phys. 2013, 214, 159–174. [Google Scholar] [CrossRef]
- Rosenboom, J.-G.; Langer, R.; Traverso, G. Bioplastics for a circular economy. Nat. Rev. Mater. 2022, 7, 117–137. [Google Scholar] [CrossRef]
- Dubey, S.P.; Thakur, V.K.; Krishnaswamy, S.; Abhyankar, H.A.; Marchante, V.; Brighton, J.L. Progress in environmental-friendly polymer nanocomposite material from PLA: Synthesis, processing and applications. Vacuum 2017, 146, 655–663. [Google Scholar] [CrossRef] [Green Version]
- European Bioplastics—Nova-Institute. Bioplastics Market Development Update 2020. Available online: http://www.european-bioplastics.org/news/publications/%0A (accessed on 30 April 2022).
- Chinthapalli, R.; Skoczinski, P.; Carus, M.; Baltus, W.; de Guzman, D.; Käb, H.; Raschka, A.; Ravenstijn, J. Biobased Building Blocks and Polymers—Global Capacities, Production and Trends, 2018–2023. Ind. Biotechnol. 2019, 15, 237–241. [Google Scholar] [CrossRef]
- Li, G.; Zhao, M.; Xu, F.; Yang, B.; Li, X.; Meng, X.; Teng, L.; Sun, F.; Li, Y. Synthesis and Biological Application of Polylactic Acid. Molecules 2020, 25, 5023. [Google Scholar] [CrossRef]
- Inkinen, S.; Hakkarainen, M.; Albertsson, A.-C.; Södergård, A. From Lactic Acid to Poly(lactic acid) (PLA): Characterization and Analysis of PLA and Its Precursors. Biomacromolecules 2011, 12, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Sreekumar, K.; Bindhu, B.; Veluraja, K. Perspectives of polylactic acid from structure to applications. Polym. Renew. Resour. 2021, 12, 60–74. [Google Scholar] [CrossRef]
- Masutani, K.; Kimura, Y. PLA Synthesis. From the Monomer to the Polymer. In Poly(lactic acid) Science and Technology: Processing, Properties, Additives and Applications; Polymer Chemistry Series; CRC Press: Boca Raton, FL, USA, 2014; Chapter 1; pp. 1–36. [Google Scholar] [CrossRef]
- Shen, L.; Worrell, E.; Patel, M. Present and future development in plastics from biomass. Biofuels Bioprod. Biorefining. 2010, 4, 25–40. [Google Scholar] [CrossRef]
- Li, Y.; Bhagwat, S.S.; Cortés-Peña, Y.R.; Ki, D.; Rao, C.V.; Jin, Y.-S.; Guest, J.S. Sustainable Lactic Acid Production from Lignocellulosic Biomass. ACS Sustain. Chem. Eng. 2021, 9, 1341–1351. [Google Scholar]
- Datta, R.; Henry, M. Lactic acid: Recent advances in products, processes and technologies—A review. J. Chem. Technol. Biotechnol. 2006, 81, 1119–1129. [Google Scholar] [CrossRef]
- Auras, R.A.; Lim, L.-T.; Selke, S.E.M.; Tsuji, H. (Eds.) Poly(lactic acid): Synthesis, Structures, Properties, Processing, and Applications; John Wiley & Sons: New York, NY, USA, 2010. [Google Scholar]
- Tang, S.-C.; Yang, J.-H. Dual Effects of Alpha-Hydroxy Acids on the Skin. Molecules 2018, 23, 863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kornhauser, A. Applications of hydroxy acids: Classification, mechanisms, and photoactivity. Clin. Cosmet. Investig. Dermatol. 2010, 3, 135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harmsen, P.F.H.; Hackmann, M.M.; Bos, H.L. Green building blocks for bio-based plastics, Biofuels. Bioprod. Biorefining 2014, 8, 306–324. [Google Scholar] [CrossRef]
- El-Sheshtawy, H.S.; Fahim, I.; Hosny, M.; El-Badry, M.A. Optimization of lactic acid production from agro-industrial wastes produced by Kosakonia cowanii. Curr. Res. Green Sustain. Chem. 2022, 5, 100228. [Google Scholar] [CrossRef]
- Jem, K.J.; Tan, B. The development and challenges of poly (lactic acid) and poly (glycolic acid). Adv. Ind. Eng. Polym. Res. 2020, 3, 60–70. [Google Scholar] [CrossRef]
- Balla, E.; Daniilidis, V.; Karlioti, G.; Kalamas, T.; Stefanidou, M.; Bikiaris, N.D.; Vlachopoulos, A.; Koumentakou, I.; Bikiaris, D.N. Poly(lactic Acid): A Versatile Biobased Polymer for the Future with Multifunctional Properties—From Monomer Synthesis, Polymerization Techniques and Molecular Weight Increase to PLA Applications. Polymers 2021, 13, 1822. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.A.C.; Balciunas, E.M.; Salgado, J.M.; González, J.M.D.; Converti, A.; de Oliveira, R.P. Lactic acid properties, applications and production: A review. Trends Food Sci. Technol. 2013, 30, 70–83. [Google Scholar] [CrossRef]
- Jem, K.J.; van der Pol, J.F.; de Vos, S. Microbial Lactic Acid, Its Polymer Poly(lactic acid), and Their Industrial Applications; Springer: Berlin/Heidelberg, Germany, 2010; pp. 323–346. [Google Scholar] [CrossRef]
- Chen, G.G.-Q. (Ed.) Plastics from Bacteria; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar] [CrossRef]
- Dusselier, M.; van Wouwe, P.; Dewaele, A.; Makshina, E.; Sels, B.F. Lactic acid as a platform chemical in the biobased economy: The role of chemocatalysis. Energy Environ. Sci. 2013, 6, 1415. [Google Scholar] [CrossRef]
- Juodeikiene, G.; Vidmantiene, D.; Basinskiene, L.; Cernauskas, D.; Bartkiene, E.; Cizeikiene, D. Green metrics for sustainability of biobased lactic acid from starchy biomass vs. chemical synthesis. Catal. Today 2015, 239, 11–16. [Google Scholar] [CrossRef]
- Rawoof, S.A.A.; Kumar, P.S.; Vo, D.-V.N.; Devaraj, K.; Mani, Y.; Devaraj, T.; Subramanian, S. Production of optically pure lactic acid by microbial fermentation: A review. Environ. Chem. Lett. 2021, 19, 539–556. [Google Scholar] [CrossRef]
- Sudhakar, M.P.; Dharani, G. Evaluation of seaweed for the production of lactic acid by fermentation using Lactobacillus plantarum. Bioresour. Technol. Rep. 2022, 17, 100890. [Google Scholar] [CrossRef]
- Cubas-Cano, E.; González-Fernández, C.; Ballesteros, M.; Tomás-Pejó, E. Biotechnological advances in lactic acid production by lactic acid bacteria: Lignocellulose as novel substrate. Biofuels Bioprod. Biorefining 2018, 12, 290–303. [Google Scholar] [CrossRef]
- Abdel-Rahman, M.A.; Sonomoto, K. Opportunities to overcome the current limitations and challenges for efficient microbial production of optically pure lactic acid. J. Biotechnol. 2016, 236, 176–192. [Google Scholar] [CrossRef]
- Kwan, T.H.; Hu, Y.; Lin, C.S.K. Techno-economic analysis of a food waste valorisation process for lactic acid, lactide and poly(lactic acid) production. J. Clean. Prod. 2018, 181, 72–87. [Google Scholar] [CrossRef]
- Narayanan, N.; Roychoudhury, P.K.; Srivastava, A. L (+) lactic acid fermentation and its product polymerization. Electron. J. Biotechnol. 2004, 7, 167–178. [Google Scholar]
- Van Wouwe, P.; Dusselier, M.; Vanleeuw, E.; Sels, B. Lactide Synthesis and Chirality Control for Polylactic acid Production. ChemSusChem 2016, 9, 907–921. [Google Scholar] [CrossRef]
- Auras, R.; Harte, B.; Selke, S. An Overview of Polylactides as Packaging Materials. Macromol. Biosci. 2004, 4, 835–864. [Google Scholar] [CrossRef]
- Gupta, A.P.; Kumar, V. New emerging trends in synthetic biodegradable polymers—Polylactide: A critique. Eur. Polym. J. 2007, 43, 4053–4074. [Google Scholar] [CrossRef]
- Jamshidian, M.; Tehrany, E.A.; Imran, M.; Jacquot, M.; Desobry, S. Poly-Lactic Acid: Production, applications, nanocomposites, and release studies. Compr. Rev. Food Sci. Food Saf. 2010, 9, 552–571. [Google Scholar] [CrossRef] [PubMed]
- Södergård, A.; Stolt, M. Properties of lactic acid based polymers and their correlation with composition. Prog. Polym. Sci. Polym. Sci. 2002, 27, 1123–1163. [Google Scholar] [CrossRef]
- Byers, J.A.; Biernesser, A.B.; Chiaie, K.R.D.; Kaur, A.; Kehl, J.A. Catalytic Systems for the Production of Poly(Lactic Acid); Springer: Cham, Switzerland, 2017; pp. 67–118. [Google Scholar] [CrossRef]
- Dusselier, M.; van Wouwe, P.; Dewaele, A.; Jacobs, P.A.; Sels, B.F. Shape-selective zeolite catalysis for bioplastics production. Science 2015, 349, 78–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, H.W.; Chang, Y.K. Economically Efficient Synthesis of Lactide Using a Solid Catalyst. Org. Process Res. Dev. 2017, 21, 1980–1984. [Google Scholar] [CrossRef]
- De Clercq, R.; Dusselier, M.; Poleunis, C.; Debecker, D.P.; Giebeler, L.; Oswald, S.; Makshina, E.; Sels, B.F. Titania-Silica Catalysts for Lactide Production from Renewable Alkyl Lactates: Structure-Activity Relations. ACS Catal. 2018, 8, 8130–8139. [Google Scholar] [CrossRef]
- Upare, P.P.; Yoon, J.W.; Hwang, D.W.; Lee, U.H.; Hwang, Y.K.; Hong, D.Y.; Kim, J.C.; Lee, J.H.; Kwak, S.K. Design of a heterogeneous catalytic process for the continuous and direct synthesis of lactide from lactic acid. Green Chem. 2016, 18, 5978–5983. [Google Scholar] [CrossRef]
- Ghadamyari, M.; Chaemchuen, S.; Zhou, K.; Dusselier, M.; Sels, B.F.; Mousavi, B.; Verpoort, F. One-step synthesis of stereo-pure L,L lactide from L-lactic acid. Catal. Commun. 2018, 114, 33–36. [Google Scholar] [CrossRef]
- Huang, Q.; Li, R.; Fu, G.; Jiang, J. Size Effects of the Crystallite of ZSM-5 Zeolites on the Direct Catalytic Conversion of L-Lactic Acid to L, L-Lactide. Crystals 2020, 10, 781. [Google Scholar] [CrossRef]
- Vert, M. After soft tissues, bone, drug delivery and packaging, PLA aims at blood. Eur. Polym. J. 2015, 68, 516–525. [Google Scholar] [CrossRef]
- Vert, M.; Chen, J.; Hellwich, K.H.; Hodge, P.; Nakano, T.; Scholz, C.; Slomkowski, S.; Vohlidal, J. Nomenclature and terminology for linear lactic acid-based polymers (IUPAC Recommendations 2019). Pure Appl. Chem. 2020, 92, 193–211. [Google Scholar] [CrossRef] [Green Version]
- Jin, F.-L.; Hu, R.-R.; Park, S.-J. Improvement of thermal behaviors of biodegradable poly(lactic acid) polymer: A review. Compos. Part B Eng. 2019, 164, 287–296. [Google Scholar] [CrossRef]
- Sin, L.T.; Bee Soo Tueen, B.S. Overview of Biodegradable Polymers and Poly(Lactic Acid). In Polylactic Acid: A Practical Guide for the Processing, Manufacturing, and Applications of PLA (Plastics Design Library), 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 97–133. [Google Scholar] [CrossRef]
- Garlotta, D. A Literature Review of Poly(Lactic Acid). J. Polym. Environ. 2001, 9, 63–83. [Google Scholar] [CrossRef]
- Zhang, C. Biodegradable Polyesters: Synthesis, Properties, Applications. In Biodegradable Polyesters; Fakirov, S., Ed.; Wiley: Hoboken, NJ, USA, 2015; Chapter 1; pp. 1–19. [Google Scholar]
- Tsuji, H. Poly(Lactic Acid). In Bio-Based Plastic; Kabasci, S., Ed.; John Wiley & Sons Ltd.: Chichester, UK, 2013; pp. 171–239. [Google Scholar] [CrossRef]
- Kulkarni, R.K. Polylactic Acid for Surgical Implants. Arch. Surg. 1966, 93, 839. [Google Scholar] [CrossRef]
- Ahmed, T.; Shahid, M.; Azeem, F.; Rasul, I.; Shah, A.A.; Noman, M.; Hameed, A.; Manzoor, N.; Manzoor, I.; Muhammad, S. Biodegradation of plastics: Current scenario and future prospects for environmental safety. Environ. Sci. Pollut. Res. 2018, 25, 7287–7298. [Google Scholar] [CrossRef]
- Taib, N.-A.A.B.; Rahman, M.R.; Huda, D.; Kuok, K.K.; Hamdan, S.; Bakri, M.K.B.; Julaihi, M.R.M.B.; Khan, A. A review on poly lactic acid (PLA) as a biodegradable polymer. Polym. Bull. 2022, 1, 1–35. [Google Scholar] [CrossRef]
- Masutani, K.; Kimura, Y. Present situation and future perspectives of poly(lactic acid). Adv. Polym. Sci. 2018, 279, 1–25. [Google Scholar]
- Choudhury, A.K.R. Sustainable Chemical Technologies for Textile Production; Elsevier Ltd.: Amsterdam, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Hu, Y.; Daoud, W.A.; Cheuk, K.K.L.; Lin, C.S.K. Newly developed techniques on polycondensation, ring-opening polymerization and polymer modification: Focus on poly(lactic acid). Materials 2016, 9, 133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ajioka, M.; Enomoto, K.; Suzuki, K.; Yamaguchi, A. Basic Properties of Polylactic Acid Produced by the Direct Condensation Polymerization of Lactic Acid. Bull. Chem. Soc. Jpn. 1995, 68, 2125–2131. [Google Scholar] [CrossRef]
- Pang, X.; Zhuang, X.; Tang, Z.; Chen, X. Polylactic acid (PLA): Research, development and industrialization. Biotechnol. J. 2010, 5, 1125–1136. [Google Scholar] [CrossRef]
- Sengupta, S.; Manna, S.; Roy, U.; Das, P. Manufacturing of Biodegradable Poly Lactic Acid (PLA): Green Alternatives to Petroleum Derived Plastics. In Encyclopedia of Renewable and Sustainable Materials; Elsevier: Amsterdam, The Netherlands, 2020; pp. 561–569. [Google Scholar] [CrossRef]
- Singhvi, M.S.; Zinjarde, S.S.; Gokhale, D.V. Polylactic acid: Synthesis and biomedical applications. J. Appl. Microbiol. 2019, 127, 1612–1626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, Y.; Deng, S.; Chen, P.; Ruan, R. Polylactic acid (PLA) synthesis and modifications: A review. Front. Chem. China 2009, 4, 259–264. [Google Scholar] [CrossRef]
- Drumright, R.E.; Gruber, P.R.; Henton, D.E. Polylactic Acid Technology. Adv. Mater. 2000, 12, 1841–1846. [Google Scholar] [CrossRef]
- Thongchul, N. Production of Lactic Acid and Polylactic Acid for Industrial Applications. In Bioprocessing Technologies in Biorefinery for Sustainable Production of Fuels, Chemicals, and Polymers; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2013; pp. 293–316. [Google Scholar] [CrossRef]
- Stanford, M.J.; Dove, A.P. Stereocontrolled ring-opening polymerisation of lactide. Chem. Soc. Rev. 2010, 39, 486–494. [Google Scholar] [CrossRef]
- Fambri, L.; Migliaresi, C. Crystallization and Thermal Properties. In Poly(Lactic Acid): Synthesis, Structures, Properties, Processing, and Applications; Auras, R., Lim, L.-T., Selke, S.E.M., Tsuji, H., Eds.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2010; pp. 113–124. [Google Scholar] [CrossRef]
- Nofar, M.; Sacligil, D.; Carreau, P.J.; Kamal, M.R.; Heuzey, M.C. Poly (lactic acid) blends: Processing, properties and applications. Int. J. Biol. Macromol. 2019, 125, 307–360. [Google Scholar] [CrossRef]
- Lasprilla, A.J.R.; Martinez, G.A.R.; Lunelli, B.H.; Jardini, A.L.; Filho, R.M. Poly-lactic acid synthesis for application in biomedical devices—A review. Biotechnol. Adv. 2012, 30, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Nampoothiri, M.K.; Nair, N.R.; John, R.P. An overview of the recent developments in polylactide (PLA) research. Bioresour. Technol. 2010, 101, 8493–8501. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Luo, Y.; Tian, X.; Huang, D.; Reddy, N.; Yang, Y. Chemical Structure of Poly(Lactic Acid). In Poly(Lactic Acid): Synthesis, Structures, Properties, Processing, and Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; pp. 69–82. [Google Scholar] [CrossRef]
- Ikada, Y.; Jamshidi, K.; Tsuji, H.; Hyon, S.H. Stereocomplex Formation between Enantiomeric Poly(lactides). Macromolecules 1987, 20, 904–906. [Google Scholar] [CrossRef]
- Ren, Q.; Wu, M.; Weng, Z.; Zhu, X.; Li, W.; Huang, P.; Wang, L.; Zheng, W.; Ohshima, M. Promoted formation of stereocomplex in enantiomeric poly(lactic acid)s induced by cellulose nanofibers. Carbohydr. Polym. 2022, 276, 118800. [Google Scholar] [CrossRef]
- Nouri, S.; Dubois, C.; Lafleur, P.G. Homocrystal and stereocomplex formation behavior of polylactides with different branched structures. Polymer 2015, 67, 227–239. [Google Scholar] [CrossRef]
- Bao, J.; Chang, R.; Shan, G.; Bao, Y.; Pan, P. Promoted Stereocomplex Crystallization in Supramolecular Stereoblock Copolymers of Enantiomeric Poly(Lactic Acid)s. Cryst. Growth Des. 2016, 16, 1502–1511. [Google Scholar] [CrossRef]
- Fukushima, K.; Hirata, M.; Kimura, Y. Synthesis and Characterization of Stereoblock Poly(lactic acid)s with Nonequivalent D/L Sequence Ratios. Macromolecules 2007, 40, 3049–3055. [Google Scholar] [CrossRef]
- Fukushima, K.; Kimura, Y. Stereocomplexed polylactides (Neo-PLA) as high-performance bio-based polymers: Their formation, properties, and application. Polym. Int. 2006, 55, 626–642. [Google Scholar] [CrossRef]
- Hirata, M.; Kimura, Y. Thermomechanical properties of stereoblock poly(lactic acid)s with different PLLA/PDLA block compositions. Polymer 2008, 49, 2656–2661. [Google Scholar] [CrossRef]
- Tsuji, H. Poly(lactide) stereocomplexes: Formation, structure, properties, degradation, and applications. Macromol. Biosci. 2005, 5, 569–597. [Google Scholar] [CrossRef]
- Tsuji, H. Poly(lactic acid) stereocomplexes: A decade of progress. Adv. Drug Deliv. Rev. 2016, 107, 97–135. [Google Scholar] [CrossRef]
- Brzeziński, M.; Biela, T. Micro- and nanostructures of polylactide stereocomplexes and their biomedical applications. Polym. Int. 2015, 64, 1667–1675. [Google Scholar] [CrossRef]
- Bai, H.; Deng, S.; Bai, D.; Zhang, Q.; Fu, Q. Recent Advances in Processing of Stereocomplex-Type Polylactide. Macromol. Rapid Commun. 2017, 38, 1700454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Tan, B.H.; Lin, T.; He, C. Recent advances in stereocomplexation of enantiomeric PLA-based copolymers and applications. Prog. Polym. Sci. 2016, 62, 22–72. [Google Scholar] [CrossRef]
- Work, W.J.; Horie, K.; Hess, M.; Stepto, R.F.T. Definition of terms related to polymer blends, composites, and multiphase polymeric materials (IUPAC Recommendations 2004). Pure Appl. Chem. 2004, 76, 1985–2007. [Google Scholar] [CrossRef]
- Yu, L.; Dean, K.; Li, L. Polymer blends and composites from renewable resources. Prog. Polym. Sci. 2006, 31, 576–602. [Google Scholar] [CrossRef]
- Saini, P.; Arora, M.; Kumar, M.N.V.R. Poly(lactic acid) blends in biomedical applications. Adv. Drug Deliv. Rev. 2016, 107, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Hamad, K.; Kaseem, M.; Ayyoob, M.; Joo, J.; Deri, F. Polylactic acid blends: The future of green, light and tough. Prog. Polym. Sci. 2018, 85, 83–127. [Google Scholar] [CrossRef]
- Bhadra, J.; Alkareem, A.; Al-Thani, N. A review of advances in the preparation and application of polyaniline based thermoset blends and composites. J. Polym. Res. 2020, 27, 122. [Google Scholar] [CrossRef] [Green Version]
- Murariu, M.; Dubois, P. PLA composites: From production to properties. Adv. Drug Deliv. Rev. 2016, 107, 17–46. [Google Scholar] [CrossRef]
- Ashothaman, A.; Sudha, J.; Senthilkumar, N. A comprehensive review on biodegradable polylactic acid polymer matrix composite material reinforced with synthetic and natural fibers. Mater. Today Proc. 2022; in press. [Google Scholar] [CrossRef]
- Xu, K.; Kozluca, A.; Denkbaş, E.B.; Pişkın, E. Synthesis of PDLLA homopolymers with different molecular weights. J. Appl. Polym. Sci. 1996, 59, 561–563. [Google Scholar] [CrossRef]
- Gao, Q.; Lan, P.; Shao, H.; Hu, X. Direct Synthesis with Melt Polycondensation and Microstructure Analysis of Poly(L-lactic acid-co-glycolic acid). Polym. J. 2002, 34, 786–793. [Google Scholar] [CrossRef] [Green Version]
- Chafran, L.S.; Campos, J.M.C.; Santos, J.S.; Sales, M.J.A.; Dias, S.C.L.; Dias, J.A. Synthesis of poly(lactic acid) by heterogeneous acid catalysis from d,l-lactic acid. J. Polym. Res. 2016, 23, 107. [Google Scholar] [CrossRef]
- Chafran, L.S.; Paiva, M.F.; França, J.O.C.; Sales, M.J.A.; Dias, S.C.L.; Dias, J.A. Preparation of PLA blends by polycondensation of D,L-lactic acid using supported 12-tungstophosphoric acid as a heterogeneous catalyst. Heliyon 2019, 5, e01810. [Google Scholar] [CrossRef] [Green Version]
- Zaky, M.S.; Wirotius, A.-L.; Coulembier, O.; Guichard, G.; Taton, D. A chiral thiourea and a phosphazene for fast and stereoselective organocatalytic ring-opening-polymerization of racemic lactide. Chem. Commun. 2021, 57, 3777–3780. [Google Scholar] [CrossRef]
- Orhan, B.; Tschan, M.J.-L.; Wirotius, A.-L.; Dove, A.P.; Coulembier, O.; Taton, D. Isoselective Ring-Opening Polymerization of rac-Lactide from Chiral Takemoto’s Organocatalysts: Elucidation of Stereocontrol. ACS Macro Lett. 2018, 7, 1413–1419. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.; Zhao, N.; Li, Z. Stereoselective Ring-Opening Polymerization of rac/Lactide Catalyzed by Squaramide Derived Organocatalysts at Room Temperature. Chin. J. Chem. 2021, 39, 2403–2409. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Z.; Wang, J.; Mai, H.; Yan, B.; Yang, F. Direct synthesis of poly(D,L-lactic acid) by melt polycondensation and its application in drug delivery. J. Appl. Polym. Sci. 2004, 91, 2143–2150. [Google Scholar] [CrossRef]
- Hosseyni, R.; Pooresmaeil, M.; Namazi, H. Star-shaped polylactic acid-based triazine dendrimers: The catalyst type and time factors influence on polylactic acid molecular weight. Iran. Polym. J. 2020, 29, 423–432. [Google Scholar] [CrossRef]
- Gierej, A.; Vagenende, M.; Filipkowski, A.; Siwicki, B.; Buczynski, R.; Thienpont, H.; van Vlierberghe, S.; Geernaert, T.; Dubruel, P.; Berghmans, F. Poly(D,L-Lactic Acid) (PDLLA) Biodegradable and Biocompatible Polymer Optical Fiber. J. Light. Technol. 2019, 37, 1916–1923. [Google Scholar] [CrossRef]
- Wu, L.; Park, J.; Kamaki, Y.; Kim, B. Optimization of the fused deposition modeling-based fabrication process for polylactic acid microneedles. Microsyst. Nanoeng. 2021, 7, 58. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Gao, Y.; Xiong, J.; Shao, W.; Cui, C.; Sun, N.; Zhang, Y.; Chang, S.; Han, P.; Liu, F.; et al. Biodegradable and high-performance multiscale structured nanofiber membrane as mask filter media via poly(lactic acid) electrospinning. J. Colloid Interface Sci. 2022, 606, 961–970. [Google Scholar] [CrossRef]
- Chaubey, A.; Aadil, K.R.; Jha, H. Synthesis and characterization of lignin-poly lactic acid film as active food packaging material. Mater. Technol. 2021, 36, 585–593. [Google Scholar] [CrossRef]
- Ciarfaglia, N.; Laezza, A.; Lods, L.; Lonjon, A.; Dandurand, J.; Pepe, A.; Bochicchio, B. Thermal and dynamic mechanical behavior of poly(lactic acid) (PLA)-based electrospun scaffolds for tissue engineering. J. Appl. Polym. Sci. 2021, 138, 51313. [Google Scholar] [CrossRef]
- Arunagiri, V.; Prasannan, A.; Udomsin, J.; Lai, J.-Y.; Wang, C.-F.; Hong, P.-D.; Tsai, H.C. Facile fabrication of eco-friendly polycaprolactone (PCL)/Poly-D, L-Lactic acid (PDLLA) modified melamine sorbent for oil-spill cleaning and water/oil (W/O) emulsion separation. Sep. Purif. Technol. 2021, 259, 118081. [Google Scholar] [CrossRef]
- Tien, N.-D.; Nishikawa, Y.; Hashimoto, M.; Tosaka, M.; Sasaki, S.; Sakurai, S. Three-dimensional analyses of spherulite morphology in poly(oxyethylene) and its blends with amorphous poly(d,l-lactic acid) using X-ray computerized tomography. Polym. J. 2015, 47, 37–44. [Google Scholar] [CrossRef]
- De Arenaza, I.M.; Meaurio, E.; Sarasu, J.-R. Analysis of the Miscibility of Polymer Blends Through Molecular Dynamics Simulation. In Polymerization; IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef] [Green Version]
- Pini, R.; Storti, G.; Mazzotti, M.; Tai, H.; Shakesheff, K.M.; Howdle, S.M. Sorption and swelling of poly(DL-lactic acid) and poly(lactic-co-glycolic acid) in supercritical CO2: An experimental and modeling study. J. Polym. Sci. Part B Polym. Phys. 2008, 46, 483–496. [Google Scholar] [CrossRef]
- Li, H.; Chang, J. Preparation and characterization of bioactive and biodegradable Wollastonite/poly(D,L-lactic acid) composite scaffolds. J. Mater. Sci. Mater. Med. 2004, 15, 1089–1095. [Google Scholar] [CrossRef]
- Mikos, A.G.; Thorsen, A.J.; Czerwonka, L.A.; Bao, Y.; Langer, R.; Winslow, D.N.; Vacanti, J.P. Preparation and characterization of poly(l-lactic acid) foams. Polymer 1994, 35, 1068–1077. [Google Scholar] [CrossRef]
- Sun, Q.; Sheng, J.; Yang, R. Controllable biodegradation and drug release behavior of chitosan-graft-poly(D, L-lactic acid) synthesized by an efficient method. Polym. Degrad. Stab. 2021, 186, 109458. [Google Scholar] [CrossRef]
- Wang, Z.-Y.; Li, X.-W.; Li, J.-N.; Li, G.-M.; Tao, J.-Q. Synthesis of poly(lactic acid)-poly(phenyl phosphate) via direct polycondensation and its characterization. J. Polym. Res. 2009, 16, 255–261. [Google Scholar] [CrossRef]
- Wang, Z.-Y.; Zhao, H.-J.; Wang, Q.-F.; Ye, R.-R.; Finlow, D.E. Synthesis of poly(D,L-lactic acid) modified by cholic acid via direct melt copolycondensation and its characterization. J. Appl. Polym. Sci. 2010, 117, 1405–1415. [Google Scholar] [CrossRef]
- Xu, W.; Sasaki, M.; Niidome, T. Sirolimus Release from Biodegradable Polymers for Coronary Stent Application: A Review. Pharmaceutics 2022, 14, 492. [Google Scholar] [CrossRef]
- Gomes, A.J.; Espreafico, E.M.; Tfouni, E. Trans-[Ru(NO)Cl(cyclam)](PF 6) 2 and [Ru(NO)(Hedta)] Incorporated in PLGA Nanoparticles for the Delivery of Nitric Oxide to B16-F10 Cells: Cytotoxicity and Phototoxicity. Mol. Pharm. 2013, 10, 3544–3554. [Google Scholar] [CrossRef]
- Lunardi, C.N.; Gomes, A.J.; Palepu, S.; Galwaduge, P.T.; Hillman, E.M.C. PLGA nano/microparticles loaded with cresyl violet as a tracer for drug delivery: Characterization and in-situ hyperspectral fluorescence and 2-photon localization. Mater. Sci. Eng. C 2017, 70, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Liu, Y.; Ma, R.; Chen, J.; Qiu, J.; Du, S.; Li, C.; Wu, Z.; Yang, X.; Chen, Z.; et al. Thermosensitive Hydrogel Incorporating Prussian Blue Nanoparticles Promotes Diabetic Wound Healing via ROS Scavenging and Mitochondrial Function Restoration. ACS Appl. Mater. Interfaces 2022, 14, 14059–14071. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.G.; Boulton, A.J.M.; Bus, S.A. Diabetic Foot Ulcers and Their Recurrence. N. Engl. J. Med. 2017, 376, 2367–2375. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, A.C.; Semeano, A.T.S.; Dourado, A.H.B.; Ulrich, H.; de Torresi, S.I.C. Novel Conducting and Biodegradable Copolymers with Noncytotoxic Properties toward Embryonic Stem Cells. ACS Omega 2018, 3, 5593–5604. [Google Scholar] [CrossRef] [PubMed]
- da Silva, A.; Minadeo, M.; de Torresi, S. Gold Nanoparticles and [PEDOT-Poly(D,L-Lactic Acid)] Composite: Synthesis, Characterization and Application to H2O2 Sensing. J. Braz. Chem. Soc. 2019, 30, 2066–2075. [Google Scholar] [CrossRef]
- Ayyoob, M.; Yang, X.; Park, H.-J.; Park, S.; Kim, J.H.; Nam, S.W.; Kim, Y.J. Synthesis of Bioresorbable Poly(Lactic-co-Glycolic Acid)s Through Direct Polycondensation: An Economical Substitute for the Synthesis of Polyglactin via ROP of Lactide and Glycolide. Fibers Polym. 2019, 20, 887–895. [Google Scholar] [CrossRef]
- Yang, J.; Yan, J.; Zhou, Z.; Amsden, B.G. Dithiol-PEG-PDLLA Micelles: Preparation and Evaluation as Potential Topical Ocular Delivery Vehicle. Biomacromolecules 2014, 15, 1346–1354. [Google Scholar] [CrossRef]
- Shi, K.; Wang, Y.-L.; Qu, Y.; Liao, J.-F.; Chu, B.-Y.; Zhang, H.-P.; Luo, F.; Qian, Z.-Y. Synthesis, characterization and application of reversible PDLLA-PEG-PDLLA copolymer thermogels in vitro and in vivo. Sci. Rep. 2016, 6, 19077. [Google Scholar] [CrossRef]
- Lee, C.W.; Nakamura, S.; Kimura, Y. Synthesis and characterization of polytulipalin-g-polylactide copolymers. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 1111–1119. [Google Scholar] [CrossRef]
- Toshikj, N.; Robin, J.-J.; Blanquer, S. A simple and general approach for the synthesis of biodegradable triblock copolymers by organocatalytic ROP from poly(lactide) macroinitiators. Eur. Polym. J. 2020, 127, 109599. [Google Scholar] [CrossRef]
- Chu, B.; Zhang, L.; Qu, Y.; Chen, X.; Peng, J.; Huang, Y.; Qian, Z. Synthesis, characterization and drug loading property of Monomethoxy-Poly(ethylene glycol)-Poly(ε-caprolactone)-Poly(D,L-lactide) (MPEG-PCLA) copolymers. Sci. Rep. 2016, 6, 34069. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, K.; Mishra, A.K.; Patel, V.K.; Vishwakarma, N.K.; Biswas, C.S.; Paira, T.K.; Mandal, T.K.; Maiti, P.; Misra, N.; Ray, B. Synthesis of well-defined amphiphilic poly(d,l-lactide)-b-poly(N-vinylpyrrolidone) block copolymers using ROP and xanthate-mediated RAFT polymerization. Polymer 2012, 53, 5743–5753. [Google Scholar] [CrossRef]
- Aluthge, D.C.; Xu, C.; Othman, N.; Noroozi, N.; Hatzikiriakos, S.G.; Mehrkhodavandi, P. PLA-PHB-PLA Triblock Copolymers: Synthesis by Sequential Addition and Investigation of Mechanical and Rheological Properties. Macromolecules 2013, 46, 3965–3974. [Google Scholar] [CrossRef]
- Sitompul, J.; Setyawan, D.; Kim, D.Y.J.; Lee, H.W. Synthesis of PDLLA/PLLA-Bentonite Nanocomposite through Sonication; AIP Publishing LLC: Melville, NY, USA, 2016; p. 020080. [Google Scholar] [CrossRef] [Green Version]
- Mohammadi, M.S.; Rezabeigi, E.; Bertram, J.; Marelli, B.; Gendron, R.; Nazhat, S.N.; Bureau, M.N. Poly(d,l-Lactic acid) Composite Foams Containing Phosphate Glass Particles Produced via Solid-State Foaming Using CO2 for Bone Tissue Engineering Applications. Polymers 2020, 12, 231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, B.; Li, H.; Yao, Q.; Zhang, Y.; Zhu, X.; Xia, T.; Wang, J.; Li, G.; Li, X.; Ni, S. Local in vitro delivery of rapamycin from electrospun PEO/PDLLA nanofibers for glioblastoma treatment. Biomed. Pharmacother. 2016, 83, 1345–1352. [Google Scholar] [CrossRef] [PubMed]
- Tudorachi, N.; Chiriac, A.P.; Mustata, F. New nanocomposite based on poly(lactic-co-glycolic acid) copolymer and magnetite. Synthesis and characterization. Compos. Part B Eng. 2015, 72, 150–159. [Google Scholar] [CrossRef]
- Song, A.; Ji, S.; Hong, J.S.; Ji, Y.; Gokhale, A.A.; Lee, I. Encapsulation of hydrophobic or hydrophilic iron oxide nanoparticles into poly(lactic acid) micro/nanoparticles via adaptable emulsion setup. J. Appl. Polym. Sci. 2016, 133, 43749. [Google Scholar] [CrossRef]
- Nkansah, M.K.; Thakral, D.; Shapiro, E.M. Magnetic poly(lactide-co-glycolide) and cellulose particles for MRI-based cell tracking. Magn. Reson. Med. 2011, 65, 1776–1785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lagarrigue, P.; Darcos, V.; Tenailleau, C.; Duployer, B.; Dupret-Bories, A.; Cazalbou, S.; Poquillon, D.; Grossin, D.; Combes, C.; Soulié, J. Poly(d,l-lactide)-Grafted Bioactive Glass Nanoparticles: From Nanobricks to Freeze-Cast Scaffolds for Bone Substitution. ACS Appl. Nano Mater. 2022, 5, 5278–5291. [Google Scholar] [CrossRef]
- Nerantzaki, M.; Prokopiou, L.; Bikiaris, D.N.; Patsiaoura, D.; Chrissafis, K.; Klonos, P.; Kyritsis, A.; Pissis, P. In situ prepared poly(DL-lactic acid)/silica nanocomposites: Study of molecular composition, thermal stability, glass transition and molecular dynamics. Thermochim. Acta 2018, 669, 16–29. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, X.; Xia, S.; Zhao, J. Cu(II) adsorption on Poly(Lactic Acid) Microplastics: Significance of microbial colonization and degradation. Chem. Eng. J. 2022, 429, 132306. [Google Scholar] [CrossRef]
- Fan, Y.-B.; Li, P.; Zeng, L.; Huang, X.-J. Effects of mechanical load on the degradation of poly(d,l-lactic acid) foam. Polym. Degrad. Stab. 2008, 93, 677–683. [Google Scholar] [CrossRef]
- Li, R.-Y.; Liu, Z.-G.; Liu, H.-Q.; Chen, L.; Liu, J.-F.; Pan, Y.-H. Evaluation of biocompatibility and toxicity of biodegradable poly (DL-lactic acid) films. Am. J. Transl. Res. 2015, 7, 1357–1370. [Google Scholar] [PubMed]















Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de França, J.O.C.; da Silva Valadares, D.; Paiva, M.F.; Dias, S.C.L.; Dias, J.A. Polymers Based on PLA from Synthesis Using D,L-Lactic Acid (or Racemic Lactide) and Some Biomedical Applications: A Short Review. Polymers 2022, 14, 2317. https://doi.org/10.3390/polym14122317
de França JOC, da Silva Valadares D, Paiva MF, Dias SCL, Dias JA. Polymers Based on PLA from Synthesis Using D,L-Lactic Acid (or Racemic Lactide) and Some Biomedical Applications: A Short Review. Polymers. 2022; 14(12):2317. https://doi.org/10.3390/polym14122317
Chicago/Turabian Stylede França, Juliene Oliveira Campos, Deborah da Silva Valadares, Mateus Freitas Paiva, Sílvia Cláudia Loureiro Dias, and José Alves Dias. 2022. "Polymers Based on PLA from Synthesis Using D,L-Lactic Acid (or Racemic Lactide) and Some Biomedical Applications: A Short Review" Polymers 14, no. 12: 2317. https://doi.org/10.3390/polym14122317
APA Stylede França, J. O. C., da Silva Valadares, D., Paiva, M. F., Dias, S. C. L., & Dias, J. A. (2022). Polymers Based on PLA from Synthesis Using D,L-Lactic Acid (or Racemic Lactide) and Some Biomedical Applications: A Short Review. Polymers, 14(12), 2317. https://doi.org/10.3390/polym14122317