Occupational Safety Analysis for COVID-Instigated Repurposed Manufacturing Lines: Use of Nanomaterials in Injection Moulding
Abstract
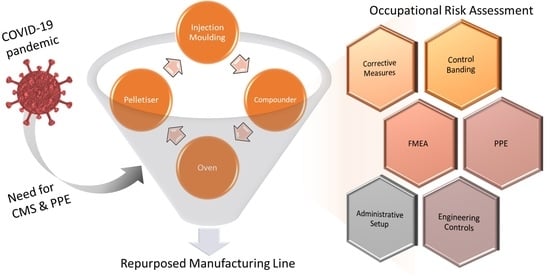
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Process for Preparation of Nanocomposite Materials
2.3. Failure Mode and Effect Analysis (FMEA)
- Severity (S)—ranked from 1 to 5; associated with the most serious effect for a given failure mode (1: least serious, 5: most serious).
- Occurrence (O)—ranked from 1 to 5; associated with the likelihood that the failure mode cause will be present in the item being analysed (1: unlikely, 5: most likely).
- Detection (D)—ranked from 1 to 5; associated with the likelihood that the failure mode can be detected and prevented based on the current process controls (1: most likely to be detected, 5: most unlikely to be detected).
2.4. Control Banding
3. Results
3.1. FMEA Risk Analysis
3.2. Nanomaterial Risk Assessment
- Ag nanoparticles were handled in dry powder form. Most commercial products of Ag nanoparticles that would be used within a repurposing-line context are available to purchase in dry powder form. It should be noted that the use of a suspension would lower the exposure risk but is impractical because of the requirement for incorporation in the thermoplastic.
- A low quantity of nanomaterials was required for each process task (approx. 10 g per 1 kg masterbatch).
- Small Ag nanoparticle size (<50 nm) was required for antimicrobial action.
- The masterbatch manufacturing process could have lasted several hours; however, the precise phase of nanoparticle handling and introduction was shorter in duration (up to 30 min per batch production).
- More than one employee was required to perform the task.
- Scenario A—No controls applied (inherent process risk);
- Scenario B—Local exhaust ventilation and PPE applied;
- Scenario C—Local exhaust ventilation and source containment and PPE applied.
4. Discussion
4.1. Prioritization of the Failures and Associated Risks
4.2. Hierarchy of Protective Measures for Repurposed Injection Moulding Units
4.3. Limitations of Study and Potential for Further Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Coates, P.D.; Whiteside, B.R.; Martyn, M.T.; Spares, R.; Gough, T. Micromoulding—Precision Processing for Controlled Products. In 4M 2006—Second International Conference on Multi-Material Micro Manufacture; Menz, W., Dimov, S., Fillon, B., Eds.; Elsevier: Oxford, UK, 2006; pp. 13–15. ISBN 978-0-08-045263-0. [Google Scholar]
- Fitzgerald, A.; McDonald, P.; Devine, D.; Fuenmayor, E. Transfer and Optimisation of Injection Moulding Manufacture of Medical Devices Using Scientific Moulding Principles. J. Manuf. Mater. Process. 2021, 5, 113. [Google Scholar] [CrossRef]
- Papadakis, L.; Avraam, S.; Photiou, D.; Masurtschak, S.; Pereira Falcón, J.C. Use of a Holistic Design and Manufacturing Approach to Implement Optimized Additively Manufactured Mould Inserts for the Production of Injection-Moulded Thermoplastics. J. Manuf. Mater. Process. 2020, 4, 100. [Google Scholar] [CrossRef]
- Battisti, M.G.; Friesenbichler, W. Injection-Moulding Compounding of PP Polymer Nanocomposites. Stroj. Vestn.—J. Mech. Eng. 2013, 59, 662–668. [Google Scholar] [CrossRef] [Green Version]
- Monti, M.; Zaccone, M.; Frache, A.; Torre, L.; Armentano, I. Dielectric Spectroscopy of PP/MWCNT Nanocomposites: Relationship with Crystalline Structure and Injection Molding Condition. Nanomaterials 2021, 11, 550. [Google Scholar] [CrossRef]
- Mourad, A.-H.I.; Mozumder, M.S.; Mairpady, A.; Pervez, H.; Kannuri, U.M. On the Injection Molding Processing Parameters of HDPE-TiO2 Nanocomposites. Materials 2017, 10, 85. [Google Scholar] [CrossRef] [Green Version]
- Phua, Y.J.; Mohd Ishak, Z.A.; Senawi, R. Injection Molded Short Glass and Carbon Fibers Reinforced Polycarbonate Hybrid Composites: Effects of Fiber Loading. J. Reinf. Plast. Compos. 2010, 29, 2592–2603. [Google Scholar] [CrossRef]
- Hassan, T.; Salam, A.; Khan, A.; Khan, S.U.; Khanzada, H.; Wasim, M.; Khan, M.Q.; Kim, I.S. Functional Nanocomposites and Their Potential Applications: A Review. J. Polym. Res. 2021, 28, 36. [Google Scholar] [CrossRef]
- Hilliou, L.; Covas, J.A. Chapter 5—Production and Processing of Polymer-Based Nanocomposites. In Nanomaterials for Food Packaging; Cerqueira, M.Â.P.R., Lagaron, J.M., Pastrana Castro, L.M., de Oliveira Soares Vicente, A.A.M., Eds.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2018; pp. 111–146. ISBN 9780323512718. [Google Scholar]
- Froggett, S.J.; Clancy, S.F.; Boverhof, D.R.; Canady, R.A. A Review and Perspective of Existing Research on the Release of Nanomaterials from Solid Nanocomposites. Part. Fibre Toxicol. 2014, 11, 17. [Google Scholar] [CrossRef] [Green Version]
- Ibn-Mohammed, T.; Mustapha, K.B.; Godsell, J.; Adamu, Z.; Babatunde, K.A.; Akintade, D.D.; Acquaye, A.; Fujii, H.; Ndiaye, M.M.; Yamoah, F.A.; et al. A Critical Analysis of the Impacts of COVID-19 on the Global Economy and Ecosystems and Opportunities for Circular Economy Strategies. Resour. Conserv. Recycl. 2021, 164, 105169. [Google Scholar] [CrossRef]
- Liu, W.; Beltagui, A.; Ye, S. Accelerated Innovation through Repurposing: Exaptation of Design and Manufacturing in Response to COVID-19. RD Manag. 2021, 51, 410–426. [Google Scholar] [CrossRef]
- Fu, H.; Xu, H.; Liu, Y.; Yang, Z.; Kormakov, S.; Wu, D.; Sun, J. Overview of Injection Molding Technology for Processing Polymers and Their Composites. ES Mater. Manuf. 2020, 8, 3–23. [Google Scholar] [CrossRef]
- Tay, J.K.; Cross, G.B.; Sun, L.; Chia, A.; Chee, J.; Loh, J.; Lim, Z.Y.; Ngiam, N.; Khang, W.P.; Yeap, S.; et al. Clinical Diagnostic Study of a Novel Injection Molded Swab for SARS-Cov-2 Testing. Infect. Dis. Ther. 2021, 10, 1015–1022. [Google Scholar] [CrossRef]
- Oladapo, B.I.; Ismail, S.O.; Afolalu, T.D.; Olawade, D.B.; Zahedi, M. Review on 3D Printing: Fight against COVID-19. Mater. Chem. Phys. 2021, 258, 123943. [Google Scholar] [CrossRef]
- Sztorch, B.; Brząkalski, D.; Jałbrzykowski, M.; Przekop, R.E. Processing Technologies for Crisis Response on the Example of COVID-19 Pandemic—Injection Molding and FFF Case Study. Processes 2021, 9, 791. [Google Scholar] [CrossRef]
- Kapoor, K.; Bigdeli, A.Z.; Dwivedi, Y.K.; Raman, R. How Is COVID-19 Altering the Manufacturing Landscape? A Literature Review of Imminent Challenges and Management Interventions. Ann. Oper. Res. 2021, 1–33. [Google Scholar] [CrossRef]
- Poduval, A.; Ayyagari, M.S.; Malinda, M.; Vimal, K.E.K.; Kumar, A.; Kandasamy, J. Barriers in Repurposing an Existing Manufacturing Plant: A Total Interpretive Structural Modeling (TISM) Approach. Oper. Manag. Res. 2021, 1–26. [Google Scholar] [CrossRef]
- ISO 20430:2020; Plastics and Rubber Machines—Injection Moulding Machines—Safety Requirements. International Organization for Standardization: Geneva, Switzerland, 2020.
- ISO 13857:2019; Safety of Machiner—Safety Distances to Prevent Hazard Zones Being Reached by Upper and Lower Limbs. International Organization for Standardization: Geneva, Switzerland, 2019.
- ISO 7010:2019; Graphical Symbols—Safety Colours and Safety Signs—Registered Safety Signs. International Organization for Standardization: Geneva, Switzerland, 2019.
- HSE. Health and Safety Executive HSE Information Sheet—Safety at Injection Moulding Machines; HSE: London, UK, 2015.
- Occupational Safety and Health Administration ETool: Machine Guarding—Plastics Machinery—Horizontal Injection Molding Machines—Safety Tour. Available online: https://www.osha.gov/etools/machine-guarding/plastics-machinery/horizontal-injection-molding-machines/safety-tour (accessed on 27 April 2022).
- ISO/TR 12885:2018; Nanotechnologies—Health and Safety Practices in Occupational Settings. International Organization for Standardization: Geneva, Switzerland, 2018.
- Warheit, D.B. Hazard and Risk Assessment Strategies for Nanoparticle Exposures: How Far Have We Come in the Past 10 Years? F1000Research 2018, 7, 376. [Google Scholar] [CrossRef] [Green Version]
- Yokel, R.A.; MacPhail, R.C. Engineered Nanomaterials: Exposures, Hazards, and Risk Prevention. J. Occup. Med. Toxicol. 2011, 6, 7. [Google Scholar] [CrossRef] [Green Version]
- Oberdörster, G.; Oberdörster, E.; Oberdörster, J. Nanotoxicology: An Emerging Discipline Evolving from Studies of Ultrafine Particles. Environ. Health Perspect. 2005, 113, 823–839. [Google Scholar] [CrossRef]
- Sengul, A.B.; Asmatulu, E. Toxicity of Metal and Metal Oxide Nanoparticles: A Review. Environ. Chem. Lett. 2020, 18, 1659–1683. [Google Scholar] [CrossRef]
- Medici, S.; Peana, M.; Pelucelli, A.; Zoroddu, M.A. An Updated Overview on Metal Nanoparticles Toxicity. Semin. Cancer Biol. 2021, 76, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Bondarenko, O.; Juganson, K.; Ivask, A.; Kasemets, K.; Mortimer, M.; Kahru, A. Toxicity of Ag, CuO and ZnO Nanoparticles to Selected Environmentally Relevant Test Organisms and Mammalian Cells in Vitro: A Critical Review. Arch. Toxicol. 2013, 87, 1181–1200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koivisto, A.J.; Kling, K.I.; Levin, M.; Fransman, W.; Gosens, I.; Cassee, F.R.; Jensen, K.A. First Order Risk Assessment for Nanoparticle Inhalation Exposure during Injection Molding of Polypropylene Composites and Production of Tungsten-Carbide-Cobalt Fine Powder Based upon Pulmonary Inflammation and Surface Area Dose. NanoImpact 2017, 6, 30–38. [Google Scholar] [CrossRef] [Green Version]
- ISO/TR 22293:2021; Evaluation of Methods for Assessing the Release of Nanomaterials from Commercial, Nanomaterial-Containing Polymer Composites. International Organization for Standardization: Geneva, Switzerland, 2021.
- Lithner, D.; Larsson, Å.; Dave, G. Environmental and Health Hazard Ranking and Assessment of Plastic Polymers Based on Chemical Composition. Sci. Total Environ. 2011, 409, 3309–3324. [Google Scholar] [CrossRef]
- Pelclova, D.; Zdimal, V.; Komarc, M.; Vlckova, S.; Fenclova, Z.; Ondracek, J.; Schwarz, J.; Kostejn, M.; Kacer, P.; Dvorackova, S.; et al. Deep Airway Inflammation and Respiratory Disorders in Nanocomposite Workers. Nanomaterials 2018, 8, 731. [Google Scholar] [CrossRef] [Green Version]
- Boonruksa, P.; Bello, D.; Zhang, J.; Isaacs, J.A.; Mead, J.L.; Woskie, S.R. Exposures to Nanoparticles and Fibers during Injection Molding and Recycling of Carbon Nanotube Reinforced Polycarbonate Composites. J. Expo. Sci. Environ. Epidemiol. 2017, 27, 379–390. [Google Scholar] [CrossRef]
- ImPURE. Available online: https://www.impure-project.eu/ (accessed on 16 May 2022).
- Palza, H. Antimicrobial Polymers with Metal Nanoparticles. Int. J. Mol. Sci. 2015, 16, 2099–2116. [Google Scholar] [CrossRef] [Green Version]
- Kadiyala, U.; Kotov, N.A.; VanEpps, J.S. Antibacterial Metal Oxide Nanoparticles: Challenges in Interpreting the Literature. Curr. Pharm. Des. 2018, 24, 896–903. [Google Scholar] [CrossRef]
- Zuniga, J.M.; Cortes, A. The Role of Additive Manufacturing and Antimicrobial Polymers in the COVID-19 Pandemic. Expert Rev. Med. Devices 2020, 17, 477–481. [Google Scholar] [CrossRef]
- Porcari, A.; Borsella, E.; Benighaus, C.; Grieger, K.; Isigonis, P.; Chakravarty, S.; Kines, P.; Jensen, K.A. From Risk Perception to Risk Governance in Nanotechnology: A Multi-Stakeholder Study. J. Nanoparticle Res. 2019, 21, 245. [Google Scholar] [CrossRef] [Green Version]
- Marin, S.; Vlasceanu, G.M.; Tiplea, R.E.; Bucur, I.R.; Lemnaru, M.; Marin, M.M.; Grumezescu, A.M. Applications and Toxicity of Silver Nanoparticles: A Recent Review. Curr. Top. Med. Chem. 2015, 15, 1596–1604. [Google Scholar] [CrossRef]
- J1739_202101; Potential Failure Mode and Effects Analysis (FMEA) Including Design FMEA, Supplemental FMEA-MSR, and Process FMEA. Society of Automotive Engineering: Warrendale, PE, USA, 2021.
- Cavaignac, A.L.D.O.; Uchoa, J.G.L. Obtaining FMEA’s Indices for Occupational Safety in Civil Construction: A Theoretical Contribution. Braz. J. Oper. Prod. Manag. 2018, 15, 558–565. [Google Scholar] [CrossRef] [Green Version]
- De Cavaignac, A.L.O.; Uchoa, J.G.L.; dos Santos, H.F.O. Risk Ananlysis and Prioritization in Water Supply Network Maintenance Work through the Failure Modes and Effects Analysis: Occupational Ssafety FMEA Application. Braz. J. Oper. Prod. Manag. 2020, 17, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Uchoa, J.G.L.; de Sousa, M.J.A.; Silva, L.H.V.; Cavaignac, A.L.D.O. FMEA Method Application Based on Occupational Risks in the Construction Industry on Work at Height: A Theoretical Contribution. Int. J. Adv. Eng. Res. Sci. 2019, 6, 261–278. [Google Scholar] [CrossRef]
- Oomen, A.G.; Steinhäuser, K.G.; Bleeker, E.A.J.; van Broekhuizen, F.; Sips, A.; Dekkers, S.; Wijnhoven, S.W.P.; Sayre, P.G. Risk Assessment Frameworks for Nanomaterials: Scope, Link to Regulations, Applicability, and Outline for Future Directions in View of Needed Increase in Efficiency. NanoImpact 2018, 9, 1–13. [Google Scholar] [CrossRef]
- Liguori, B.; Hansen, S.F.; Baun, A.; Jensen, K.A. Control Banding Tools for Occupational Exposure Assessment of Nanomaterials—Ready for Use in a Regulatory Context? NanoImpact 2016, 2, 1–17. [Google Scholar] [CrossRef] [Green Version]
- ISO/TS 12901-2:2014; Nanotechnologies—Occupational Risk Management Applied to Engineered Nanomaterials—Part 2: Use of the Control Banding Approach. International Organization for Standardization: Geneva, Switzerland, 2014.
- Van Duuren-Stuurman, B.; Vink, S.R.; Verbist, K.J.M.; Heussen, H.G.A.; Brouwer, D.H.; Kroese, D.E.D.; Van Niftrik, M.F.J.; Tielemans, E.; Fransman, W. Stoffenmanager Nano Version 1.0: A Web-Based Tool for Risk Prioritization of Airborne Manufactured Nano Objects. Ann. Occup. Hyg. 2012, 56, 525–541. [Google Scholar] [CrossRef]
- National Institute for Occupational Safety and Health. Workplace Design Solutions: Protecting Workers during Intermediate and Downstream Processing of Nanomaterials; National Institute for Occupational Safety and Health: Autorta, CO, USA, 2018.
- Gliga, A.R.; Skoglund, S.; Odnevall Wallinder, I.; Fadeel, B.; Karlsson, H.L. Size-Dependent Cytotoxicity of Silver Nanoparticles in Human Lung Cells: The Role of Cellular Uptake, Agglomeration and Ag Release. Part. Fibre Toxicol. 2014, 11, 11. [Google Scholar] [CrossRef] [Green Version]
- Niska, K.; Knap, N.; Kędzia, A.; Jaskiewicz, M.; Kamysz, W.; Inkielewicz-Stepniak, I. Capping Agent-Dependent Toxicity and Antimicrobial Activity of Silver Nanoparticles: An In Vitro Study. Concerns about Potential Application in Dental Practice. Int. J. Med. Sci. 2016, 13, 772–782. [Google Scholar] [CrossRef] [Green Version]
- Tsai, S.-J.C.; Ashter, A.; Ada, E.; Mead, J.L.; Barry, C.F.; Ellenbecker, M.J. Control of Airborne Nanoparticles Release during Compounding of Polymer Nanocomposites. Nano 2008, 03, 301–309. [Google Scholar] [CrossRef]
- Theriault, M.; Yoeuth, S.; Matar, J.; Martin, J.; Bello, D.; Barry, C. Investigation of Nanoparticles Emitted When Injection Molding Neat and Additive-Filled Polypropylene and Polycarbonate. In Proceedings of the AIP Conference Proceedings; American Institute of Physics: College Park, MD, USA, 15 December 2017; Volume 1914, p. 140008. [Google Scholar]
- Stephens, B.; Azimi, P.; El Orch, Z.; Ramos, T. Ultrafine Particle Emissions from Desktop 3D Printers. Atmos. Environ. 2013, 79, 334–339. [Google Scholar] [CrossRef]
- Mansfield, E.; Kaiser, D.L.; Fujita, D.; Van de Voorde, M. Metrology and Standardization for Nanotechnology: Protocols and Industrial Innovations; Wiley: Hoboken, NJ, USA, 2017; ISBN 978-3-527-34039-2. [Google Scholar]
- Ferdous, Z.; Nemmar, A. Health Impact of Silver Nanoparticles: A Review of the Biodistribution and Toxicity Following Various Routes of Exposure. Int. J. Mol. Sci. 2020, 21, 2375. [Google Scholar] [CrossRef] [Green Version]
- Keren, N.; West, H.H.; Rogers, W.J.; Gupta, P.J.; Mannan, M.S. Use of Failure Rate Databases and Process Safety Performance Measurements to Improve Process Safety; Mary Kay O’Connor Process Safety Center: College Station, TX, USA, 2002. [Google Scholar]
- Cockshott, J.E. Probability Bow-Ties: A Transparent Risk Management Tool. Process Saf. Environ. Prot. 2005, 83, 307–316. [Google Scholar] [CrossRef] [Green Version]
- Gjerstad, T. OREDA—The Reliability Data Reference for the Offshore Industry. Presented at the SPE Offshore Europe, Aberdeen, UK, 10–13 September 1985. [Google Scholar]
- Trotta, T.; Kashou, C.; Faulk, N. Pressure Relief Valve Inspection Interval. Process Saf. Prog. 2018, 37, 37–41. [Google Scholar] [CrossRef]
- Raafat, H.M.N. Comparative Strategy for the Safety of Horizontal Injection Moulding Machines. Saf. Sci. 1993, 16, 67–88. [Google Scholar] [CrossRef]
- Institut de Recherche Robert-Sauvé en Santé et en Sécurité du Travail (IRSST). Plastic Injection Moulding Machines with Auxiliary Equipment—Safety During Maintenance and Production Interventions. Available online: https://www.irsst.qc.ca/en/publications-tools/publication/i/100753/n/presses-a-injection-plastique (accessed on 17 May 2022).
- Jones, S.; Kirchsteiger, C.; Bjerke, W. The Importance of near Miss Reporting to Further Improve Safety Performance. J. Loss Prev. Process Ind. 1999, 12, 59–67. [Google Scholar] [CrossRef]
- Health and Safety Commission. Control of Substances Hazardous to Health: The Control of Substances Hazardous to Health Regulations 2002; Health and Safety Executive: Sudbury, ON, Canada, 2005; ISBN 9780717629817.
- Shahrubudin, N.; Lee, T.C.; Ramlan, R. An Overview on 3D Printing Technology: Technological, Materials, and Applications. Procedia Manuf. 2019, 35, 1286–1296. [Google Scholar] [CrossRef]
- Davis, A.Y.; Zhang, Q.; Wong, J.P.S.; Weber, R.J.; Black, M.S. Characterization of Volatile Organic Compound Emissions from Consumer Level Material Extrusion 3D Printers. Build. Environ. 2019, 160, 106209. [Google Scholar] [CrossRef]
- Li, A.J.; Pal, V.K.; Kannan, K. A Review of Environmental Occurrence, Toxicity, Biotransformation and Biomonitoring of Volatile Organic Compounds. Environ. Chem. Ecotoxicol. 2021, 3, 91–116. [Google Scholar] [CrossRef]
- Zitting, A.; Savolainen, H. Effects of Single and Repeated Exposures to Thermo-Oxidative Degradation Products of Poly(Acrylonitrile-Butadiene-Styrene) (ABS) on Rat Lung, Liver, Kidney, and Brain. Arch. Toxicol. 1980, 46, 295–304. [Google Scholar] [CrossRef]
- Dematteo, R.; Keith, M.M.; Brophy, J.T.; Wordsworth, A.; Watterson, A.E.; Beck, M.; Ford, A.R.; Gilbertson, M.; Pharityal, J.; Rootham, M.; et al. Chemical Exposures of Women Workers in the Plastics Industry with Particular Reference to Breast Cancer and Reproductive Hazards. New Solut. J. Environ. Occup. Health Policy 2013, 22, 427–448. [Google Scholar] [CrossRef] [PubMed]
- Rudel, R.A.; Attfield, K.R.; Schifano, J.N.; Brody, J.G. Chemicals Causing Mammary Gland Tumors in Animals Signal New Directions for Epidemiology, Chemicals Testing, and Risk Assessment for Breast Cancer Prevention. Cancer 2007, 109, 2635–2666. [Google Scholar] [CrossRef] [PubMed]
- Czeizel, A.E.; Hegedüs, S.; Tímár, L. Congenital Abnormalities and Indicators of Germinal Mutations in the Vicinity of an Acrylonitrile Producing Factory. Mutat. Res. Mol. Mech. Mutagen. 1999, 427, 105–123. [Google Scholar] [CrossRef]
- Brody, J.G.; Moysich, K.B.; Humblet, O.; Attfield, K.R.; Beehler, G.P.; Rudel, R.A. Environmental Pollutants and Breast Cancer. Cancer 2007, 109, 2667–2711. [Google Scholar] [CrossRef]
- Owen, P.E.; Glaister, J.R.; Gaunt, I.F.; Pullinger, D.H. Inhalation Toxicity Studies With 1,3-Butadiene 3 Two Year Toxicity/Carcinogenicity Study in Rats. Am. Ind. Hyg. Assoc. J. 1987, 48, 407–413. [Google Scholar] [CrossRef]
- ISO 45001:2018; Occupational Health and Safety Management Systems—Requirements with Guidance for Use. International Organization for Standardization: Geneva, Switzerland, 2018.
- ASHRAE. Classification of Laboratory Ventilation Design Levels; ASHRAE Technical Committee 9.10, Laboratory Systems, Ed.; ASHRAE: Atlanta, GA, USA, 2018. [Google Scholar]
- Directorate-General for Employment, European Agency for Safty and Health at Work. Working Safely with Manufactured Nanomaterials: Non Binding Guide for Workers; Publications Office of the European Union: Luxemburg, 2019; ISBN 9789279464621. [Google Scholar]
- Health and Safety Executive. Controlling Noise at Work: The Control of Noise at Rork Regulations 2005, Guidance on Regulations; HSE Books: Norwich, UK, 2021; ISBN 9780717665679.
- Jensen, K.A.; Saber, A.T.; Kristensen, H.V.; Koponen, I.K.; Liguori, B.; Wallin, H. NanoSafer vs. 1.1—Nanomaterial Risk Assessment Using First Order Modeling. In Proceedings of the 6th International Symposium on Nanotechnology, Occupational and Environmental Health, Nagoya, Japan, 28–30 October 2013. [Google Scholar]
- Zalk, D.; Paik, S.; Swuste, P. Control Banding Nanotool: Evaluation of a Qualitative Risk Assessment Method for the Control of Nanoparticulate Exposures; International Congress on Occupational Health: Cape Town, South Africa, 2009. [Google Scholar]
- Eastlake, A.C.; Beaucham, C.; Martinez, K.F.; Dahm, M.M.; Sparks, C.; Hodson, L.L.; Geraci, C.L. Refinement of the Nanoparticle Emission Assessment Technique into the Nanomaterial Exposure Assessment Technique (NEAT 2.0). J. Occup. Environ. Hyg. 2016, 13, 708–717. [Google Scholar] [CrossRef] [Green Version]
- ENV/JM/MONO(2015)19; Harmonized Tiered Approach to Measure and Assess the Potential Exposure to Airborne Emissions of Engineered Nano-Objects and Their Agglomerates and Aggregates at Workplaces. Organisation for Economic Co-operation and Development: Paris, France, 2015.
- Macdonald, D. Practical Hazops, Trips and Alarms, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2004; ISBN 9780080480190. [Google Scholar]
- Chandra Ray, P.; Yu, H.; Fu, P.P. Toxicity and Environmental Risks of Nanomaterials: Challenges and Future Needs. J. Environ. Sci. Health Part C 2009, 27, 1–35. [Google Scholar] [CrossRef] [Green Version]
- Hischier, R.; Walser, T. Life Cycle Assessment of Engineered Nanomaterials: State of the Art and Strategies to Overcome Existing Gaps. Sci. Total Environ. 2012, 425, 271–282. [Google Scholar] [CrossRef]
- Lekamge, S.; Miranda, A.F.; Abraham, A.; Li, V.; Shukla, R.; Bansal, V.; Nugegoda, D. The Toxicity of Silver Nanoparticles (AgNPs) to Three Freshwater Invertebrates With Different Life Strategies: Hydra Vulgaris, Daphnia Carinata, and Paratya Australiensis. Front. Environ. Sci. 2018, 6, 152. [Google Scholar] [CrossRef]
- Gorka, D.E.; Osterberg, J.S.; Gwin, C.A.; Colman, B.P.; Meyer, J.N.; Bernhardt, E.S.; Gunsch, C.K.; DiGulio, R.T.; Liu, J. Reducing Environmental Toxicity of Silver Nanoparticles through Shape Control. Environ. Sci. Technol. 2015, 49, 10093–10098. [Google Scholar] [CrossRef]
- Meyer, D.E.; Curran, M.A.; Gonzalez, M.A. An Examination of Silver Nanoparticles in Socks Using Screening-Level Life Cycle Assessment. J. Nanopart. Res. 2011, 13, 147–156. [Google Scholar] [CrossRef]



| Level | Severity (S) | Occurrence (O) | Detection (D) |
|---|---|---|---|
| 1 | Negligible | Almost impossible | Almost certain |
| 2 | Minor | Low | High |
| 3 | Serious | Moderate | Moderate |
| 4 | Major | High | Low |
| 5 | Fatal | Almost certain | Almost impossible |
| Failure Modes | Node | Threat | Source | Description |
|---|---|---|---|---|
| FM1 | Injection Moulding | Cuts, bruises, injuries | Moving mechanical parts | Injuries caused by moulds dismantling tooling |
| FM2 | Burns | Hot surfaces | Exposed hot surfaces | |
| FM3 | Burns | Heated material | Hot specimens from the mould | |
| FM4 | Inhalation of hazardous fumes | Melted or heated polymer | ||
| FM5 | Eye irritation/damage | Dust | Dust during the final step of the injection process (relaxation) | |
| FM6 | Respiratory irritation/damage | |||
| FM7 | Explosion | High pressure | Operation at max 16 bar through a pneumatic system | |
| FM8 | Electric shock | Electrical hazards | Equipment was connected to the power grid | |
| FM9 | Hearing damage | Noise | Mainly due to the compressor (>100 dB). Disturbing periodic noise from the vent | |
| FM10 | Strain injury, injury from falling object | Lifting of heavy parts | Moulds for IM | |
| FM11 | Eruption | Compressor operation and emptying | High-pressure 90 L vessel on compressor and tubing below the flooring at approx. 15 bars | |
| FM12 | Inhalation of/dermal contact with ENMs | Handling of ENMs | Nanocomposite masterbatch production | |
| FM13 | Pelletizer | Cuts, bruises, injuries | Moving mechanical parts | Cuts during pelletizer operation (blades at high rotation speeds) |
| FM14 | Eye irritation/damage | Dust | Mainly from the pelletizer | |
| FM15 | Respiratory irritation/damage | Mainly from the pelletizer | ||
| FM16 | Electric shock | Electrical hazards | Equipment connected to the power grid | |
| FM17 | Compounder | Burns | Hot surfaces | Exposed hot surfaces |
| FM18 | Burns | Heated material | Melted polymer from the compounder | |
| FM19 | Inhalation of hazardous fumes | Due to melted or heated polymer | ||
| FM20 | Explosion | High pressure | Compounder can reach up to 80 bars | |
| FM21 | Electrical shock | Electrical hazards | Equipment connected to the power grid | |
| FM22 | Strain injury, injury from falling object | Lifting of heavy parts | Feeders for compounder | |
| FM23 | Electric shock | Water spillage | Cooling water bath for the extruded material (close to cables, sockets, etc.) | |
| FM24 | Inhalation of/dermal contact with ENMs | Handling of ENMs | Nanoparticles inserted in the compounder through a gravimetric feeder in dry form | |
| FM25 | Oven | Burns | Hot surfaces | Exposed hot surfaces |
| FM26 | Burns | Heated material | Hot pellets dried in the oven | |
| FM27 | Inhalation of hazardous fumes | Due to the heated polymer | ||
| FM28 | Electric shock | Electrical hazards | Equipment connected to the power grid |
| Failure Modes | Affected Groups | Existing Controls | S | O | D | RPN |
|---|---|---|---|---|---|---|
| FM1 | O | Standard operating procedure (SOP), heavy-duty gloves, pelletizer interlock | 2 | 4 | 3 | 24 |
| FM2 | O | SOP, heavy-duty gloves, safety cover | 3 | 4 | 1 | 12 |
| FM3 | O | SOP, lab coat, heavy-duty gloves, closed shoes | 3 | 4 | 1 | 12 |
| FM4 | O, B | Arm hood, central ventilation system, respirators | 3 | 4 | 2 | 24 |
| FM5 | O, B | Arm hood, central ventilation system, safety glasses | 2 | 2 | 3 | 12 |
| FM6 | O, B | Arm hood, central ventilation system, respirators | 2 | 2 | 3 | 12 |
| FM7 | O, B | Fire extinguisher, fire plan, safety glasses, pressure indicators on the compressor and IM machine, pressure sensors on the compounder with emergency shutdown | 5 | 1 | 2 | 10 |
| FM8 | O | Lightning arrester in the switchboard, current relay, extra grounding, power safety for the operation bench | 4 | 1 | 2 | 8 |
| FM9 | O, B | Isolation of compressor to a separate room | 3 | 5 | 1 | 15 |
| FM10 | O, B | SOP, closed shoes | 3 | 4 | 1 | 12 |
| FM11 | O, B | SOP, pressure valve and indicator | 5 | 3 | 2 | 30 |
| FM12 | O, B | Isolated inlet system: material is filled in the feeder under a fume hood and then placed on the extruder feeder. Arm hood above the feeder. PPEs (face mask, gloves, lab coat, shoe covering, hair covering) | 2 | 4 | 4 | 32 |
| FM13 | O | SOP, heavy-duty gloves, pelletizer interlock | 2 | 3 | 2 | 12 |
| FM14 | O, B | Arm hood, central ventilation system, safety glasses | 2 | 2 | 3 | 12 |
| FM15 | O, B | Arm hood, central ventilation system, respirators | 2 | 2 | 3 | 12 |
| FM16 | O | Lightning arrester in the switchboard, current relay, extra grounding, power safety for the operation bench | 4 | 1 | 2 | 8 |
| FM17 | O | SOP, safety cover, heavy-duty gloves | 3 | 4 | 1 | 12 |
| FM18 | O | SOP, lab coat, heavy-duty gloves, closed shoes | 3 | 4 | 1 | 12 |
| FM19 | O, B | Arm hood, central ventilation system, respirators | 3 | 4 | 2 | 24 |
| FM20 | O, B | Fire extinguisher, fire plan, safety glasses, pressure indicators on the compressor and the IM machine, pressure sensors on the compounder with emergency shutdown | 5 | 1 | 2 | 10 |
| FM21 | O | Lightning arrester in the switchboard, current relay, extra grounding, power safety for the operation bench | 4 | 1 | 2 | 8 |
| FM22 | O, B | SOP, closed shoes | 3 | 4 | 1 | 12 |
| FM23 | O | SOP, release valve on the bottom of the bath | 5 | 3 | 2 | 30 |
| FM24 | O, B | Isolated inlet system: material is filled in the feeder under a fume hood and then placed on the extruder feeder. Arm hood above the feeder. PPEs (face mask, gloves, lab coat, shoes covering, hair covering). Use of liquid pump to insert the particles in suspension form | 3 | 4 | 4 | 48 |
| FM25 | O | SOP, heavy-duty gloves, safety cover | 3 | 4 | 1 | 12 |
| FM26 | O | SOP, lab coat, heavy-duty gloves, closed shoes | 3 | 4 | 1 | 12 |
| FM27 | O, B | Arm hood, central ventilation system, respirators | 3 | 2 | 2 | 12 |
| FM28 | O | Lightning arrester in the switchboard, current relay, extra grounding, power safety for the operation bench | 4 | 1 | 2 | 8 |
| Stoffenmanager Nano Input Fields | Parameters Input within Tool | |
|---|---|---|
| Process Type | Handling of Bulk Aggregated/Agglomerated Powders | |
| Product Characteristics | Dustiness | Unknown |
| Moisture content | Dry product | |
| Concentration of nano component | 100% | |
| Does it contain one of the following OECD components? | Ag (nanosilver) | |
| Is the primary particle diameter larger than 50 nm? | No | |
| Handling/Process | Task characterization | Handling of products in small amounts (up to 100 g) |
| Task duration | 30–120 min a day | |
| Task frequency | 2–3 days a week | |
| Task performed within breathing zone of employee | Yes | |
| Working Area | Working room being cleaned daily; | Yes |
| inspections and maintenance of machines/ancillary equipment being done at least monthly | Yes | |
| Working room volume | 100–1000 m3 | |
| Ventilation in working room | Mechanical or natural ventilation | |
| Local Control Measures and Personal Protective Equipment | Local control measures | Scenario A—No controls Scenario B—Local exhaust ventilation Scenario C—Containment of the source with local exhaust ventilation |
| Personal protective equipment | Scenario A—No controls Scenario B—FFP3 mask Scenario C—FFP3 mask | |
| Priority Number | Failure Modes | Affected Groups | S | O | D | RPN |
|---|---|---|---|---|---|---|
| 1 | FM24 | O, B | 3 | 4 | 4 | 48 |
| 2 | FM12 | O, B | 2 | 4 | 4 | 32 |
| 3 | FM11 | O, B | 5 | 3 | 2 | 30 |
| 4 | FM23 | O | 5 | 3 | 2 | 30 |
| 5 | FM1 | O | 2 | 4 | 3 | 24 |
| 6 | FM4 | O, B | 3 | 4 | 2 | 24 |
| 7 | FM19 | O, B | 3 | 4 | 2 | 24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Damilos, S.; Saliakas, S.; Kokkinopoulos, I.; Karayannis, P.; Karamitrou, M.; Trompeta, A.-F.; Charitidis, C.; Koumoulos, E.P. Occupational Safety Analysis for COVID-Instigated Repurposed Manufacturing Lines: Use of Nanomaterials in Injection Moulding. Polymers 2022, 14, 2418. https://doi.org/10.3390/polym14122418
Damilos S, Saliakas S, Kokkinopoulos I, Karayannis P, Karamitrou M, Trompeta A-F, Charitidis C, Koumoulos EP. Occupational Safety Analysis for COVID-Instigated Repurposed Manufacturing Lines: Use of Nanomaterials in Injection Moulding. Polymers. 2022; 14(12):2418. https://doi.org/10.3390/polym14122418
Chicago/Turabian StyleDamilos, Spyridon, Stratos Saliakas, Ioannis Kokkinopoulos, Panagiotis Karayannis, Melpo Karamitrou, Aikaterini-Flora Trompeta, Costas Charitidis, and Elias P. Koumoulos. 2022. "Occupational Safety Analysis for COVID-Instigated Repurposed Manufacturing Lines: Use of Nanomaterials in Injection Moulding" Polymers 14, no. 12: 2418. https://doi.org/10.3390/polym14122418
APA StyleDamilos, S., Saliakas, S., Kokkinopoulos, I., Karayannis, P., Karamitrou, M., Trompeta, A. -F., Charitidis, C., & Koumoulos, E. P. (2022). Occupational Safety Analysis for COVID-Instigated Repurposed Manufacturing Lines: Use of Nanomaterials in Injection Moulding. Polymers, 14(12), 2418. https://doi.org/10.3390/polym14122418