Bioinspired Bottlebrush Polymers for Aqueous Boundary Lubrication
Abstract
:1. Introduction
2. Bio-Lubricants
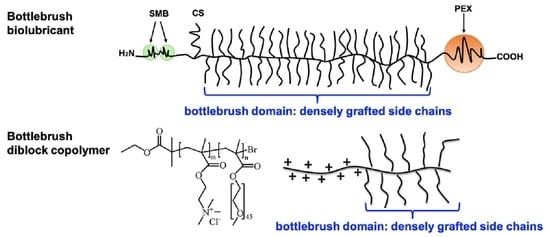
3. Bottlebrush Polymers
3.1. Synthesis of Bottlebrush Polymers
3.2. Adsorption of Bottlebrush Polyelectrolytes at Surfaces
3.3. Interfacial Lubrication Properties of Bottlebrush Polymer Layers
3.4. Random Bottlebrush Polymers
3.5. Diblock Bottlebrush Polymers
3.6. Triblock Bottlebrush Polymers
3.7. Wear Resistance Influenced by Molecular Adsorption Strength
3.8. Synergistic Aqueous Lubrication Mediated by Aggregates of Natural Molecules and Polymers
4. Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singer, I.L.; Pollock, H. Fundamentals of Friction: Macroscopic and Microscopic Processes; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Forster, H.; Fisher, J. The Influence of Loading Time and Lubricant on the Friction of Articular Cartilage. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 1996, 210, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Gale, L.R.; Coller, R.; Hargreaves, D.; Hills, B.A.; Crawford, R. The role of SAPL as a boundary lubricant in prosthetic joints. Tribol. Int. 2007, 40, 601–606. [Google Scholar] [CrossRef]
- Wright, V.; Dowson, D. Lubrication and cartilage. J. Anat. 1976, 121, 107–118. [Google Scholar]
- Hills, B.A. Surface-active phospholipid: A Pandora’s box of clinical applications. Part II. Barrier and lubricating properties. Intern. Med. J. 2002, 32, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Klein, J. Molecular mechanisms of synovial joint lubrication. Proc. Inst. Mech. Eng. Part J J. Eng. Tribol. 2006, 220, 691–710. [Google Scholar] [CrossRef]
- Levangie, P.K.; Norkin, C.C. Joint Structure and Function: A Comprehensive Analysis, 5th ed.; F. A. Davis Company: Philadelphia, PA, USA, 2011. [Google Scholar]
- Dėdinaitė, A. Biomimetic lubrication. Soft Matter 2012, 8, 273–284. [Google Scholar] [CrossRef]
- Abbasi, M.; Faust, L.; Wilhelm, M. Comb and Bottlebrush Polymers with Superior Rheological and Mechanical Properties. Adv. Mater. 2019, 31, 1806484. [Google Scholar] [CrossRef]
- Verduzco, R.; Li, X.Y.; Pesek, S.L.; Stein, G.E. Structure, function, self-assembly, and applications of bottlebrush copolymers. Chem. Soc. Rev. 2015, 44, 2405–2420. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Muller, M.; Ratoi-Salagean, M.; Vörös, J.; Pasche, S.; De Paul, S.M.; Spikes, H.A.; Textor, M.; Spencer, N.D. Boundary Lubrication of Oxide Surfaces by Poly(L-lysine)-g-poly(ethylene glycol) (PLL-g-PEG) in Aqueous Media. Tribol. Lett. 2003, 15, 231–239. [Google Scholar] [CrossRef]
- Pettersson, T.; Naderi, A.; Makuška, R.; Claesson, P.M. Lubrication Properties of Bottle-Brush Polyelectrolytes: An AFM Study on the Effect of Side Chain and Charge Density. Langmuir 2008, 24, 3336–3347. [Google Scholar] [CrossRef]
- Linse, P.; Claesson, P.M. Modeling of Bottle-Brush Polymer Adsorption onto Mica and Silica Surfaces. Macromolecules 2009, 42, 6310–6318. [Google Scholar] [CrossRef]
- Galuschko, A.; Spirin, L.; Kreer, T.; Johner, A.; Pastorino, C.; Wittmer, J.; Baschnagel, J. Frictional Forces between Strongly Compressed, Nonentangled Polymer Brushes: Molecular Dynamics Simulations and Scaling Theory. Langmuir 2010, 26, 6418–6429. [Google Scholar] [CrossRef] [PubMed]
- Spirin, L.; Galuschko, A.; Kreer, T.; Johner, A.; Baschnagel, J.; Binder, K. Polymer-brush lubrication in the limit of strong compression. Eur. Phys. J. E 2010, 33, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Russano, D.; Carrillo, J.-M.Y.; Dobrynin, A.V. Interaction between Brush Layers of Bottle-Brush Polyelectrolytes: Molecular Dynamics Simulations. Langmuir 2011, 27, 11044–11051. [Google Scholar] [CrossRef] [PubMed]
- Leermakers, F.; Zhulina, E.; Borisov, O. Interaction forces and lubrication of dendronized surfaces. Curr. Opin. Colloid Interface Sci. 2017, 27, 50–56. [Google Scholar] [CrossRef]
- Kreer, T. Polymer-brush lubrication: A review of recent theoretical advances. Soft Matter 2016, 12, 3479–3501. [Google Scholar] [CrossRef]
- Sakakibara, K.; Maeda, K.; Yoshikawa, C.; Tsujii, Y. Water Lubricating and Biocompatible Films of Bacterial Cellulose Nanofibers Surface-Modified with Densely Grafted, Concentrated Polymer Brushes. ACS Appl. Nano Mater. 2021, 4, 1503–1511. [Google Scholar] [CrossRef]
- Yan, W.; Ramakrishna, S.N.; Spencer, N.D.; Benetti, E.M. Brushes, Graft Copolymers, or Bottlebrushes? The Effect of Polymer Architecture on the Nanotribological Properties of Grafted-from Assemblies. Langmuir 2019, 35, 11255–11264. [Google Scholar] [CrossRef]
- Sun, Z.; Feeney, E.; Guan, Y.; Cook, S.G.; Gourdon, D.; Bonassar, L.J.; Putnam, D. Boundary mode lubrication of articular cartilage with a biomimetic diblock copolymer. Proc. Natl. Acad. Sci. USA 2019, 116, 12437–12441. [Google Scholar] [CrossRef] [Green Version]
- Adibnia, V.; Olszewski, M.; De Crescenzo, G.; Matyjaszewski, K.; Banquy, X. Superlubricity of Zwitterionic Bottlebrush Polymers in the Presence of Multivalent Ions. J. Am. Chem. Soc. 2020, 142, 14843–14847. [Google Scholar] [CrossRef]
- Jia, W.; Tian, J.; Bai, P.; Li, S.; Zeng, H.; Zhang, W.; Tian, Y. A novel comb-typed poly(oligo(ethylene glycol) methylether acrylate) as an excellent aqueous lubricant. J. Colloid Interface Sci. 2018, 539, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Fu, T.; Yue, Q.; Liu, H.; Ma, S.; Cai, M.; Zhou, F. Graphene oxide/brush-like polysaccharide copolymer nanohybrids as eco-friendly additives for water-based lubrication. Tribol. Int. 2021, 157, 106895. [Google Scholar] [CrossRef]
- Claesson, P.; Makuska, R.; Varga, I.; Meszaros, R.; Titmuss, S.; Linse, P.; Pedersen, J.S.; Stubenrauch, C. Bottle-brush polymers: Adsorption at surfaces and interactions with surfactants. Adv. Colloid Interface Sci. 2010, 155, 50–57. [Google Scholar] [CrossRef]
- Naderi, A.; Makuška, R.; Claesson, P.M. Interactions between bottle-brush polyelectrolyte layers: Effects of ionic strength and oppositely charged surfactant. J. Colloid Interface Sci. 2008, 323, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Olanya, G.; Iruthayaraj, J.; Poptoshev, E.; Makuska, R.; Vareikis, A.; Claesson, P.M. Adsorption Characteristics of Bottle-Brush Polymers on Silica: Effect of Side Chain and Charge Density. Langmuir 2008, 24, 5341–5349. [Google Scholar] [CrossRef]
- Liu, X.; Dedinaite, A.; Nylander, T.; Dabkowska, A.P.; Skoda, M.; Makuska, R.; Claesson, P.M. Association of anionic surfactant and physisorbed branched brush layers probed by neutron and optical reflectometry. J. Colloid Interface Sci. 2014, 440, 245–252. [Google Scholar] [CrossRef]
- Liu, X.; Dedinaite, A.; Rutland, M.; Thormann, E.; Visnevskij, C.; Makuska, R.; Claesson, P.M. Electrostatically Anchored Branched Brush Layers. Langmuir 2012, 28, 15537–15547. [Google Scholar] [CrossRef]
- Liu, X.; Thormann, E.; Dedinaite, A.; Rutland, M.; Visnevskij, C.; Makuska, R.; Claesson, P.M. Low friction and high load bearing capacity layers formed by cationic-block-non-ionic bottle-brush copolymers in aqueous media. Soft Matter 2013, 9, 5361–5371. [Google Scholar] [CrossRef] [Green Version]
- Müller, M.; Lee, S.; Spikes, H.A.; Spencer, N.D. The Influence of Molecular Architecture on the Macroscopic Lubrication Properties of the Brush-Like Co-polyelectrolyte Poly(L-lysine)-g-poly(ethylene glycol) (PLL-g-PEG) Adsorbed on Oxide Surfaces. Tribol. Lett. 2003, 15, 395–405. [Google Scholar] [CrossRef] [Green Version]
- Dedinaite, A.; Thormann, E.; Olanya, G.; Claesson, P.M.; Nyström, B.; Kjøniksen, A.-L.; Zhu, K. Friction in aqueous media tuned by temperature-responsive polymer layers. Soft Matter 2010, 6, 2489–2498. [Google Scholar] [CrossRef]
- Yan, X.; Perry, S.S.; Spencer, N.; Pasche, S.; De Paul, S.M.; Textor, M.; Lim, M.S. Reduction of Friction at Oxide Interfaces upon Polymer Adsorption from Aqueous Solutions. Langmuir 2003, 20, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.T.; Yan, X.; Lee, S.; Perry, S.S.; Spencer, N.D. Preferential Solvation and Its Effect on the Lubrication Properties of a Surface-Bound, Brushlike Copolymer. Macromolecules 2005, 38, 3861–3866. [Google Scholar] [CrossRef]
- Müller, M.T.; Yan, X.; Lee, S.; Perry, S.S.; Spencer, N.D. Lubrication Properties of a Brushlike Copolymer as a Function of the Amount of Solvent Absorbed within the Brush. Macromolecules 2005, 38, 5706–5713. [Google Scholar] [CrossRef]
- Lee, S.; Spencer, N.D. Poly(l-lysine)-graft-poly(ethylene glycol): A versatile aqueous lubricant additive for tribosystems involving thermoplastics. Lubr. Sci. 2007, 20, 21–34. [Google Scholar] [CrossRef]
- Hartung, W.; Drobek, T.; Lee, S.; Zürcher, S.; Spencer, N.D. The Influence of Anchoring-Group Structure on the Lubricating Properties of Brush-Forming Graft Copolymers in an Aqueous Medium. Tribol. Lett. 2008, 31, 119–128. [Google Scholar] [CrossRef]
- Lee, S.; Spencer, N.D. Adsorption Properties of Poly(l-lysine)-graft-poly(ethylene glycol) (PLL-g-PEG) at a Hydrophobic Interface: Influence of Tribological Stress, pH, Salt Concentration, and Polymer Molecular Weight. Langmuir 2008, 24, 9479–9488. [Google Scholar] [CrossRef] [PubMed]
- Perrino, C.; Lee, S.; Choi, S.W.; Maruyama, A.; Spencer, N.D. A Biomimetic Alternative to Poly(ethylene glycol) as an Antifouling Coating: Resistance to Nonspecific Protein Adsorption of Poly(l-lysine)-graft-dextran. Langmuir 2008, 24, 8850–8856. [Google Scholar] [CrossRef]
- Iruthayaraj, J.; Olanya, G.; Claesson, P.M. Viscoelastic Properties of Adsorbed Bottle-brush Polymer Layers Studied by Quartz Crystal Microbalance—Dissipation Measurements. J. Phys. Chem. C 2008, 112, 15028–15036. [Google Scholar] [CrossRef]
- Perrino, C.; Lee, S.; Spencer, N.D. End-grafted Sugar Chains as Aqueous Lubricant Additives: Synthesis and Macrotribological Tests of Poly(l-lysine)-graft-Dextran (PLL-g-dex) Copolymers. Tribol. Lett. 2008, 33, 83–96. [Google Scholar] [CrossRef]
- Bijelic, G.; Shovsky, A.; Varga, I.; Makuska, R.; Claesson, P.M. Adsorption characteristics of brush polyelectrolytes on silicon oxynitride revealed by dual polarization interferometry. J. Colloid Interface Sci. 2010, 348, 189–197. [Google Scholar] [CrossRef]
- Faivre, J.; Shrestha, B.R.; Burdynska, J.; Xie, G.; Moldovan, F.; Delair, T.; Benayoun, S.; David, L.; Matyjaszewski, K.; Banquy, X. Wear Protection without Surface Modification Using a Synergistic Mixture of Molecular Brushes and Linear Polymers. ACS Nano 2017, 11, 1762–1769. [Google Scholar] [CrossRef] [PubMed]
- Faivre, J.; Shrestha, B.R.; Xie, G.; Delair, T.; David, L.; Matyjaszewski, K.; Banquy, X. Unraveling the Correlations between Conformation, Lubrication, and Chemical Stability of Bottlebrush Polymers at Interfaces. Biomacromolecules 2017, 18, 4002–4010. [Google Scholar] [CrossRef] [PubMed]
- Faivre, J.; Montembault, A.; Sudre, G.; Shrestha, B.R.; Xie, G.; Matyjaszewski, K.; Benayoun, S.; Banquy, X.; Delair, T.; David, L. Lubrication and Wear Protection of Micro-Structured Hydrogels Using Bioinspired Fluids. Biomacromolecules 2018, 20, 326–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faivre, J.; Shrestha, B.R.; Xie, G.; Olszewski, M.; Adibnia, V.; Moldovan, F.; Montembault, A.; Sudre, G.; Delair, T.; David, L.; et al. Intermolecular Interactions between Bottlebrush Polymers Boost the Protection of Surfaces against Frictional Wear. Chem. Mater. 2018, 30, 4140–4149. [Google Scholar] [CrossRef]
- Xia, Y.; Adibnia, V.; Huang, R.; Murschel, F.; Faivre, J.; Xie, G.; Olszewski, M.; De Crescenzo, G.; Qi, W.; He, Z.; et al. Biomimetic Bottlebrush Polymer Coatings for Fabrication of Ultralow Fouling Surfaces. Angew. Chem. Int. Ed. 2019, 58, 1308–1314. [Google Scholar] [CrossRef]
- Perry, S.S.; Yan, X.; Limpoco, F.T.; Lee, S.; Müller, M.; Spencer, N.D. Tribological Properties of Poly(l-lysine)-graft-poly(ethylene glycol) Films: Influence of Polymer Architecture and Adsorbed Conformation. ACS Appl. Mater. Interfaces 2009, 1, 1224–1230. [Google Scholar] [CrossRef]
- Hartung, W.; Rossi, A.; Lee, S.; Spencer, N.D. Aqueous Lubrication of SiC and Si3N4 Ceramics Aided by a Brush-like Copolymer Additive, Poly(l-lysine)-graft-poly(ethylene glycol). Tribol. Lett. 2009, 34, 201–210. [Google Scholar] [CrossRef] [Green Version]
- Dedinaite, A.; Pettersson, T.; Mohanty, B.; Claesson, P.M. Lubrication by organized soft matter. Soft Matter 2010, 6, 1520–1526. [Google Scholar] [CrossRef]
- Krivorotova, T.; Makuška, R.; Naderi, A.; Claesson, P.; Dėdinaitė, A. Synthesis and interfacial properties of novel cationic polyelectrolytes with brush-on-brush structure of poly(ethylene oxide) side chains. Eur. Polym. J. 2010, 46, 171–180. [Google Scholar] [CrossRef]
- Moglianetti, M.; Campbell, R.A.; Nylander, T.; Varga, I.; Mohanty, B.; Claesson, P.M.; Makuška, R.; Titmuss, S. Interaction of sodium dodecyl sulfate and high charge density comb polymers at the silica/water interface. Soft Matter 2009, 5, 3646–3656. [Google Scholar] [CrossRef]
- Xu, X.; Billing, M.; Ruths, M.; Klok, H.; Yu, J. Structure and Functionality of Polyelectrolyte Brushes: A Surface Force Perspective. Chem. Asian J. 2018, 13, 3411–3436. [Google Scholar] [CrossRef] [PubMed]
- Mocny, P.; Klok, H.-A. Tribology of surface-grafted polymer brushes. Mol. Syst. Des. Eng. 2016, 1, 141–154. [Google Scholar] [CrossRef]
- Ma, L.; Gaisinskaya-Kipnis, A.; Kampf, N.; Klein, J. Origins of hydration lubrication. Nat. Commun. 2015, 6, 6060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estrella, R.P.; Whitelock, J.M.; Packer, N.H.; Karlsson, N.G. The glycosylation of human synovial lubricin: Implications for its role in inflammation. Biochem. J. 2010, 429, 359–367. [Google Scholar] [CrossRef] [Green Version]
- Swann, D.A.; Sotman, S.; Dixon, M.; Brooks, C. The isolation and partial characterization of the major glycoprotein (LGP-I) from the articular lubricating fraction from bovine synovial fluid. Biochem. J. 1977, 161, 473–485. [Google Scholar] [CrossRef] [Green Version]
- Jay, G.D.; Waller, K.A. The biology of Lubricin: Near frictionless joint motion. Matrix Biol. 2014, 39, 17–24. [Google Scholar] [CrossRef]
- Pettersson, T.; Dėdinaitė, A. Normal and friction forces between mucin and mucin–chitosan layers in absence and presence of SDS. J. Colloid Interface Sci. 2008, 324, 246–256. [Google Scholar] [CrossRef]
- Efremova, N.V.; Huang, Y.; Peppas, N.A.; Leckband, D.E. Direct Measurement of Interactions between Tethered Poly(ethylene glycol) Chains and Adsorbed Mucin Layers. Langmuir 2002, 18, 836–845. [Google Scholar] [CrossRef]
- Harvey, N.M.; Yakubov, G.E.; Stokes, J.R.; Klein, J. Normal and Shear Forces between Surfaces Bearing Porcine Gastric Mucin, a High-Molecular-Weight Glycoprotein. Biomacromolecules 2011, 12, 1041–1050. [Google Scholar] [CrossRef]
- Zappone, B.; Patil, N.J.; Madsen, J.B.; Pakkanen, K.I.; Lee, S. Molecular Structure and Equilibrium Forces of Bovine Submaxillary Mucin Adsorbed at a Solid–Liquid Interface. Langmuir 2015, 31, 4524–4533. [Google Scholar] [CrossRef]
- An, J.; Dėdinaitė, A.; Nilsson, A.; Holgersson, J.; Claesson, P.M. Comparison of a Brush-with-Anchor and a Train-of-Brushes Mucin on Poly(methyl methacrylate) Surfaces: Adsorption, Surface Forces, and Friction. Biomacromolecules 2014, 15, 1515–1525. [Google Scholar] [CrossRef]
- An, J.; Jin, C.; Dėdinaitė, A.; Holgersson, J.; Karlsson, N.G.; Claesson, P.M. Influence of Glycosylation on Interfacial Properties of Recombinant Mucins: Adsorption, Surface Forces, and Friction. Langmuir 2017, 33, 4386–4395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seog, J.; Dean, D.; Plaas, A.H.K.; Wong-Palms, S.; Grodzinsky, A.J.; Ortiz, C. Direct Measurement of Glycosaminoglycan Intermolecular Interactions via High-Resolution Force Spectroscopy. Macromolecules 2002, 35, 5601–5615. [Google Scholar] [CrossRef] [Green Version]
- Hardingham, T.E.; Fosang, A.J. Proteoglycans: Many forms and many functions. FASEB J. 1992, 6, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Dean, D.; Ortiz, C.; Grodzinsky, A.J. Lateral Nanomechanics of Cartilage Aggrecan Macromolecules. Biophys. J. 2007, 92, 1384–1398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horkay, F.; Basser, P.J.; Hecht, A.-M.; Geissler, E. Gel-like behavior in aggrecan assemblies. J. Chem. Phys. 2008, 128, 135103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldberg, R.; Schroeder, A.; Silbert, G.; Turjeman, K.; Barenholz, Y.; Klein, J. Boundary Lubricants with Exceptionally Low Friction Coefficients Based on 2D Close-Packed Phosphatidylcholine Liposomes. Adv. Mater. 2011, 23, 3517–3521. [Google Scholar] [CrossRef]
- Goldberg, R.; Schroeder, A.; Barenholz, Y.; Klein, J. Interactions between Adsorbed Hydrogenated Soy Phosphatidylcholine (HSPC) Vesicles at Physiologically High Pressures and Salt Concentrations. Biophys. J. 2011, 100, 2403–2411. [Google Scholar] [CrossRef] [Green Version]
- Goldberg, R.; Klein, J. Liposomes as lubricants: Beyond drug delivery. Chem. Phys. Lipids 2012, 165, 374–381. [Google Scholar] [CrossRef]
- Sivan, S.; Schroeder, A.; Verberne, G.; Merkher, Y.; Diminsky, D.; Priev, A.; Maroudas, A.; Halperin, G.; Nitzan, D.; Etsion, I.; et al. Liposomes Act as Effective Biolubricants for Friction Reduction in Human Synovial Joints. Langmuir 2009, 26, 1107–1116. [Google Scholar] [CrossRef]
- Dėdinaitė, A.; Wieland, D.C.F.; Bełdowski, P.; Claesson, P.M. Biolubrication synergy: Hyaluronan–Phospholipid interactions at interfaces. Adv. Colloid Interface Sci. 2019, 274, 102050. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Kampf, N.; Klein, J. Boundary Lubrication, Hemifusion, and Self-Healing of Binary Saturated and Monounsaturated Phosphatidylcholine Mixtures. Langmuir 2019, 35, 15459–15468. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Kluzek, M.; Iuster, N.; Shimoni, E.; Kampf, N.; Goldberg, R.; Klein, J. Cartilage-inspired, lipid-based boundary-lubricated hydrogels. Science 2020, 370, 335–338. [Google Scholar] [CrossRef] [PubMed]
- Tadmor, R.; Chen, N.; Israelachvili, J. Normal and Shear Forces between Mica and Model Membrane Surfaces with Adsorbed Hyaluronan. Macromolecules 2003, 36, 9519–9526. [Google Scholar] [CrossRef]
- Li, S.; Macakova, L.; Bełdowski, P.; Claesson, P.M.; Dėdinaitė, A. Phospholipids and Hyaluronan: From Molecular Interactions to Nano- and Macroscale Friction. Colloids Interfaces 2022, 6, 38. [Google Scholar] [CrossRef]
- Lapcik, L., Jr.; Lapcik, L.; De Smedt, S.; Demeester, J.; Chabreček, P. Hyaluronan: Preparation, Structure, Properties, and Applications. Chem. Rev. 1998, 98, 2663–2684. [Google Scholar] [CrossRef]
- Seror, J.; Merkher, Y.; Kampf, N.; Collinson, L.; Day, A.J.; Maroudas, A.; Klein, J. Articular Cartilage Proteoglycans As Boundary Lubricants: Structure and Frictional Interaction of Surface-Attached Hyaluronan and Hyaluronan–Aggrecan Complexes. Biomacromolecules 2011, 12, 3432–3443. [Google Scholar] [CrossRef]
- Forsey, R.W.; Fisher, J.; Thompson, J.; Stone, M.H.; Bell, C.; Ingham, E. The effect of hyaluronic acid and phospholipid based lubricants on friction within a human cartilage damage model. Biomaterials 2006, 27, 4581–4590. [Google Scholar] [CrossRef]
- Wang, M.; Liu, C.; Thormann, E.; Dėdinaitė, A. Hyaluronan and Phospholipid Association in Biolubrication. Biomacromolecules 2013, 14, 4198–4206. [Google Scholar] [CrossRef]
- Raj, A.; Wang, M.; Zander, T.; Wieland, D.F.; Liu, X.; An, J.; Garamus, V.M.; Willumeit-Römer, R.; Fielden, M.; Claesson, P.M.; et al. Lubrication synergy: Mixture of hyaluronan and dipalmitoylphosphatidylcholine (DPPC) vesicles. J. Colloid Interface Sci. 2017, 488, 225–233. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Wang, M.; An, J.; Thormann, E.; Dėdinaitė, A. Hyaluronan and phospholipids in boundary lubrication. Soft Matter 2012, 8, 10241–10244. [Google Scholar] [CrossRef]
- Spahn, G.; Wittig, R. Spannungs-und Bruchverhalten des gesunden Gelenkknorpels unter axialer Belastung. Eine biomechanische Untersuchung. Zent. Für Chir. 2003, 128, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Bełdowski, P.; Weber, P.; Dėdinaitė, A.; Claesson, P.M.; Gadomski, A. Physical crosslinking of hyaluronic acid in the presence of phospholipids in an aqueous nano-environment. Soft Matter 2018, 14, 8997–9004. [Google Scholar] [CrossRef]
- Raj, A.; Wang, M.; Liu, C.; Ali, L.; Karlsson, N.G.; Claesson, P.M.; Dėdinaitė, A. Molecular synergy in biolubrication: The role of cartilage oligomeric matrix protein (COMP) in surface-structuring of lubricin. J. Colloid Interface Sci. 2017, 495, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Dėdinaitė, A.; Claesson, P.M. Synergies in lubrication. Phys. Chem. Chem. Phys. 2017, 19, 23677–23689. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Banquy, X.; Greene, G.; Lowrey, D.D.; Israelachvili, J.N. The Boundary Lubrication of Chemically Grafted and Cross-Linked Hyaluronic Acid in Phosphate Buffered Saline and Lipid Solutions Measured by the Surface Forces Apparatus. Langmuir 2011, 28, 2244–2250. [Google Scholar] [CrossRef]
- Das, S.; Banquy, X.; Zappone, B.; Greene, G.W.; Jay, G.; Israelachvili, J.N. Synergistic Interactions between Grafted Hyaluronic Acid and Lubricin Provide Enhanced Wear Protection and Lubrication. Biomacromolecules 2013, 14, 1669–1677. [Google Scholar] [CrossRef]
- Majd, S.E.; Kuijer, R.; Köwitsch, A.; Groth, T.; Schmidt, T.A.; Sharma, P.K. Both Hyaluronan and Collagen Type II Keep Proteoglycan 4 (Lubricin) at the Cartilage Surface in a Condition That Provides Low Friction during Boundary Lubrication. Langmuir 2014, 30, 14566–14572. [Google Scholar] [CrossRef]
- Kelly, M.T.; Kent, E.W.; Zhao, B. Stepwise Conformational Transitions of Stimuli-Responsive Linear Ternary Heterografted Bottlebrush Polymers in Aqueous Solution. Macromolecules 2022, 55, 1629–1641. [Google Scholar] [CrossRef]
- Li, Z.; Tang, M.; Liang, S.; Zhang, M.; Biesold, G.M.; He, Y.; Hao, S.-M.; Choi, W.; Liu, Y.; Peng, J.; et al. Bottlebrush polymers: From controlled synthesis, self-assembly, properties to applications. Prog. Polym. Sci. 2021, 116, 101387. [Google Scholar] [CrossRef]
- Yin, L.; Liu, L.; Zhang, N. Brush-like polymers: Design, synthesis and applications. Chem. Commun. 2021, 57, 10484–10499. [Google Scholar] [CrossRef]
- Ribeiro, J.P.M.; Mendonça, P.V.; Coelho, J.F.J.; Matyjaszewski, K.; Serra, A.C. Glycopolymer Brushes by Reversible Deactivation Radical Polymerization: Preparation, Applications, and Future Challenges. Polymers 2020, 12, 1268. [Google Scholar] [CrossRef]
- Clarke, B.R.; Tew, G.N. Synthesis and characterization of poly(ethylene glycol) bottlebrush networks via ring-opening metathesis polymerization. J. Appl. Polym. Sci. 2022, 60, 1501–1510. [Google Scholar] [CrossRef]
- Wu, Y.; Tang, Q.; Zhang, M.; Li, Z.; Zhu, W.; Liu, Z. Synthesis of bottlebrush polymers with v-shaped side chains. Polymer 2018, 143, 190–199. [Google Scholar] [CrossRef] [Green Version]
- Matyjaszewski, K. Advanced Materials by Atom Transfer Radical Polymerization. Adv. Mater. 2018, 30, e1706441. [Google Scholar] [CrossRef]
- Beers, K.L.; Gaynor, S.G.; Matyjaszewski, K.; Sheiko, S.S.; Möller, M. The Synthesis of Densely Grafted Copolymers by Atom Transfer Radical Polymerization. Macromolecules 1998, 31, 9413–9415. [Google Scholar] [CrossRef]
- Lee, H.-I.; Boyce, J.R.; Nese, A.; Sheiko, S.S.; Matyjaszewski, K. pH-induced conformational changes of loosely grafted molecular brushes containing poly(acrylic acid) side chains. Polymer 2008, 49, 5490–5496. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, H.; Sun, Z.; Li, H.; Huang, H.; Liu, L.; Chen, Y. Shell of amphiphilic molecular bottlebrush matters as unimolecular micelle. Polymer 2018, 149, 316–324. [Google Scholar] [CrossRef]
- Cheng, C.; Khoshdel, E.; Wooley, K.L. Facile One-Pot Synthesis of Brush Polymers through Tandem Catalysis Using Grubbs’ Catalyst for Both Ring-Opening Metathesis and Atom Transfer Radical Polymerizations. Nano Lett. 2006, 6, 1741–1746. [Google Scholar] [CrossRef]
- Li, Y.; Themistou, E.; Zou, J.; Das, B.P.; Tsianou, M.; Cheng, C. Facile Synthesis and Visualization of Janus Double-Brush Copolymers. ACS Macro Lett. 2012, 1, 52–56. [Google Scholar] [CrossRef]
- Börner, H.G.; Beers, A.K.; Matyjaszewski, K.; Sheiko, A.S.S.; Möller, M. Synthesis of Molecular Brushes with Block Copolymer Side Chains Using Atom Transfer Radical Polymerization. Macromolecules 2001, 34, 4375–4383. [Google Scholar] [CrossRef]
- Gao, H.; Matyjaszewski, K. Synthesis of Molecular Brushes by “Grafting onto” Method: Combination of ATRP and Click Reactions. J. Am. Chem. Soc. 2007, 129, 6633–6639. [Google Scholar] [CrossRef]
- Huang, K.; Canterbury, D.P.; Rzayev, J. Organosoluble polypyrrole nanotubes from core–shell bottlebrush copolymers. Chem. Commun. 2010, 46, 6326–6328. [Google Scholar] [CrossRef]
- Jin, X.-H.; Price, M.B.; Finnegan, J.R.; Boott, C.E.; Richter, J.M.; Rao, A.; Menke, S.M.; Friend, R.H.; Whittell, G.R.; Manners, I. Long-range exciton transport in conjugated polymer nanofibers prepared by seeded growth. Science 2018, 360, 897–900. [Google Scholar] [CrossRef] [Green Version]
- Hua, Z.; Jones, J.R.; Thomas, M.; Arno, M.C.; Souslov, A.; Wilks, T.R.; O’Reilly, R.K. Anisotropic polymer nanoparticles with controlled dimensions from the morphological transformation of isotropic seeds. Nat. Commun. 2019, 10, 5406. [Google Scholar] [CrossRef]
- Foster, J.C.; Varlas, S.; Couturaud, B.; Coe, Z.; O’Reilly, R.K. Getting into Shape: Reflections on a New Generation of Cylindrical Nanostructures’ Self-Assembly Using Polymer Building Blocks. J. Am. Chem. Soc. 2019, 141, 2742–2753. [Google Scholar] [CrossRef] [Green Version]
- Pearce, A.K.; Wilks, T.R.; Arno, M.C.; O’Reilly, R.K. Synthesis and applications of anisotropic nanoparticles with precisely defined dimensions. Nat. Rev. Chem. 2020, 5, 21–45. [Google Scholar] [CrossRef]
- Ma, S.; Zhang, X.; Yu, B.; Zhou, F. Brushing up functional materials. NPG Asia Mater. 2019, 11, 24. [Google Scholar] [CrossRef]
- Yang, W.; Zhou, F. Polymer brushes for antibiofouling and lubrication. Biosurf. Biotribol. 2017, 3, 97–114. [Google Scholar] [CrossRef]
- Lin, W.; Klein, J. Recent Progress in Cartilage Lubrication. Adv. Mater. 2021, 33, 2005513. [Google Scholar] [CrossRef]
- Chen, M.; Briscoe, W.H.; Armes, S.P.; Klein, J. Lubrication at Physiological Pressures by Polyzwitterionic Brushes. Science 2009, 323, 1698–1701. [Google Scholar] [CrossRef] [Green Version]
- Wei, Q.; Cai, M.; Zhou, F.; Liu, W. Dramatically Tuning Friction Using Responsive Polyelectrolyte Brushes. Macromolecules 2013, 46, 9368–9379. [Google Scholar] [CrossRef]
- Yu, J.; Jackson, N.E.; Xu, X.; Morgenstern, Y.; Kaufman, Y.; Ruths, M.; de Pablo, J.J.; Tirrell, M. Multivalent counterions diminish the lubricity of polyelectrolyte brushes. Science 2018, 360, 1434–1438. [Google Scholar] [CrossRef] [Green Version]
- Banquy, X.; Burdyńska, J.; Lee, D.W.; Matyjaszewski, K.; Israelachvili, J. Bioinspired Bottle-Brush Polymer Exhibits Low Friction and Amontons-like Behavior. J. Am. Chem. Soc. 2014, 136, 6199–6202. [Google Scholar] [CrossRef]
- Ulcinas, A.; Valdre, G.; Snitka, V.; Miles, M.J.; Claesson, P.M.; Antognozzi, M. Shear Response of Nanoconfined Water on Muscovite Mica: Role of Cations. Langmuir 2011, 27, 10351–10355. [Google Scholar] [CrossRef]
- Feuz, L.; Leermakers, F.A.M.; Textor, M.; Borisov, O. Adsorption of Molecular Brushes with Polyelectrolyte Backbones onto Oppositely Charged Surfaces: A Self-Consistent Field Theory. Langmuir 2008, 24, 7232–7244. [Google Scholar] [CrossRef]
- Zappone, B.; Ruths, M.; Greene, G.; Jay, G.; Israelachvili, J.N. Adsorption, Lubrication, and Wear of Lubricin on Model Surfaces: Polymer Brush-Like Behavior of a Glycoprotein. Biophys. J. 2007, 92, 1693–1708. [Google Scholar] [CrossRef] [Green Version]
- Kang, T.; Banquy, X.; Heo, J.; Lim, C.; Lynd, N.A.; Lundberg, P.; Oh, D.X.; Lee, H.-K.; Hong, Y.-K.; Hwang, D.S.; et al. Mussel-Inspired Anchoring of Polymer Loops That Provide Superior Surface Lubrication and Antifouling Properties. ACS Nano 2016, 10, 930–937. [Google Scholar] [CrossRef] [Green Version]
- Sedó, J.; Saiz-Poseu, J.; Busqué, F.; Ruiz-Molina, D. Catechol-Based Biomimetic Functional Materials. Adv. Mater. 2013, 25, 653–701. [Google Scholar] [CrossRef] [Green Version]
- Levine, Z.A.; Rapp, M.V.; Wei, W.; Mullen, R.G.; Wu, C.; Zerze, G.H.; Mittal, J.; Waite, J.H.; Israelachvili, J.N.; Shea, J.-E. Surface force measurements and simulations of mussel-derived peptide adhesives on wet organic surfaces. Proc. Natl. Acad. Sci. USA 2016, 113, 4332–4337. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Müller, M.; Heeb, R.; Zürcher, S.; Tosatti, S.; Heinrich, M.; Amstad, F.; Pechmann, S.; Spencer, N.D. Self-healing behavior of a polyelectrolyte-based lubricant additive for aqueous lubrication of oxide materials. Tribol. Lett. 2006, 24, 217–223. [Google Scholar] [CrossRef] [Green Version]
- Dobryden, I.; Steponavičiūtė, M.; Klimkevičius, V.; Makuška, R.; Dėdinaitė, A.; Liu, X.; Corkery, R.W.; Claesson, P.M. Bioinspired Adhesion Polymers: Wear Resistance of Adsorption Layers. Langmuir 2019, 35, 15515–15525. [Google Scholar] [CrossRef]
- Dobryden, I.; Steponavičiūtė, M.; Hedman, D.; Klimkevičius, V.; Makuška, R.; Dėdinaitė, A.; Liu, X.; Corkery, R.W.; Claesson, P.M. Local Wear of Catechol-Containing Diblock Copolymer Layers: Wear Volume, Stick–Slip, and Nanomechanical Changes. J. Phys. Chem. C 2021, 125, 21277–21292. [Google Scholar] [CrossRef]
- Drummond, C.; Marinov, G.; Richetti, P. Reinforcement of a Surfactant Boundary Lubricant Film by a Hydrophilic−Hydrophilic Diblock Copolymer. Langmuir 2008, 24, 1560–1565. [Google Scholar] [CrossRef]







| Compositions | Substrate | Interfacial Lubricating Properties | Ref. |
|---|---|---|---|
| HA + DPPC liposomes | Damaged human cartilage | The reduction in friction was 69.5%, P = 1.3 MPa | [79] |
| HA + Aggrecan | Mica | μeff = 0.01, P = 1.6 MPa | [78] |
| HA + DPPC vesicles | Macroscopic glass surfaces | μeff = 0.1, P = 210 MPa | [77] |
| HA + DPPC bilayer | Silica | μeff = 0.03, P = 56 MPa | [80] |
| HA + DPPC vesicles | Silica | μeff < 0.01, P = 23 MPa | [81] |
| COMP + lubricin | PMMA | μeff = 0.06, P = 7 MPa | [85] |
| cross-linked HA + DOPC | Mica | μeff > 0.5, P = 2 MPa | [88] |
| HA + Lubricin | Mica | μeff = 0.09–0.4, P = 4 MPa | [89] |
| HA + Lubricin + Type II collagen | Gold versus SiO2 | μeff = 0.01, P = 0.013 MPa | [90] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Claesson, P.M. Bioinspired Bottlebrush Polymers for Aqueous Boundary Lubrication. Polymers 2022, 14, 2724. https://doi.org/10.3390/polym14132724
Liu X, Claesson PM. Bioinspired Bottlebrush Polymers for Aqueous Boundary Lubrication. Polymers. 2022; 14(13):2724. https://doi.org/10.3390/polym14132724
Chicago/Turabian StyleLiu, Xiaoyan, and Per M. Claesson. 2022. "Bioinspired Bottlebrush Polymers for Aqueous Boundary Lubrication" Polymers 14, no. 13: 2724. https://doi.org/10.3390/polym14132724
APA StyleLiu, X., & Claesson, P. M. (2022). Bioinspired Bottlebrush Polymers for Aqueous Boundary Lubrication. Polymers, 14(13), 2724. https://doi.org/10.3390/polym14132724