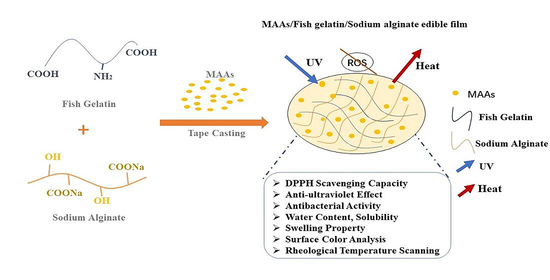
The Preparation of Anti-Ultraviolet Composite Films Based on Fish Gelatin and Sodium Alginate Incorporated with Mycosporine-like Amino Acids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction of MAAs
2.3. Characterization of MAAs
2.3.1. Ultraviolet Spectral Scanning
2.3.2. DPPH Radical Scavenging Ability
2.4. Preparation of the Composite Film
2.5. Determination of the Physical Properties of the Composite Film
2.5.1. Apparent Surface Color Analysis
2.5.2. Thickness and Ductility
2.5.3. Water Content, Swelling Degree and Water Solubility
2.5.4. Water Vapor Permeability (WVP)
2.5.5. Rheological Experiment
2.5.6. DPPH Radical Scavenging Capacity
2.5.7. Antibacterial Properties
2.5.8. Optical Blocking Performance
2.6. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Properties of MAAs
3.2. The Physicochemical Properties of Composite Films
3.2.1. Surface Color Analysis
3.2.2. Thickness and Mechanical Properties
3.2.3. Water Content, Solubility, Swelling Properties and WVP
3.2.4. Rheological Temperature Scanning
3.2.5. Antioxidant Activity
3.2.6. Antibacterial Properties
3.2.7. Optical Blocking Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, L.; Ramakanth, D.; Akhila, K.; Gaikwad, K.K. Edible films and coatings for food packaging applications: A review. Environ. Chem. Lett. 2022, 20, 875–900. [Google Scholar] [CrossRef]
- Roullier, C.; Chollet-Krugler, M.; Pferschy-Wenzig, E.; Maillard, A.; Rechberger, G.N.; Legouin-Gargadennec, B.; Bauer, R.; Boustie, J. Characterization and identification of mycosporines-like compounds in cyanolichens. Isolation of mycosporine hydroxyglutamicol from Nephroma laevigatum Ach. Phytochemistry 2011, 72, 1348–1357. [Google Scholar] [CrossRef] [PubMed]
- Carreto, J.I.; Carignan, M.O. Mycosporine-like amino acids: Relevant secondary metabolites. Chemical and ecological aspects. Mar. Drugs 2011, 9, 387–446. [Google Scholar] [CrossRef] [PubMed]
- Karsten, U.; Sawall, T.; Wiencke, C. A survey of the distribution of UV-absorbing substances in tropical macroalgae. Phycol. Res. 1998, 46, 271–279. [Google Scholar]
- Sinha, R.P.; Klisch, M.; Gröniger, A.; Häder, D.P. Ultraviolet-absorbing/screening substances in cyanobacteria, phytoplankton and macroalgae. J. Photochem. Photobiol. B Biol. 1998, 47, 83–94. [Google Scholar] [CrossRef]
- Gómez-Guillén, M.C.; Pérez-Mateos, M.; Gómez-Estaca, J.; López-Caballero, E.; Giménez, B.; Montero, P. Fish gelatin: A renewable material for developing active biodegradable films. Trends Food Sci. Technol. 2009, 20, 3–16. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Rhim, J. Preparation and application of agar/alginate/collagen ternary blend functional food packaging films. Int. J. Biol. Macromol. 2015, 80, 460–468. [Google Scholar] [CrossRef]
- Bitencourt, C.M.; Fávaro-Trindade, C.S.; Sobral, P.J.A.; Carvalho, R.A. Gelatin-based films additivated with curcuma ethanol extract: Antioxidant activity and physical properties of films. Food Hydrocoll. 2014, 40, 145–152. [Google Scholar] [CrossRef]
- Kowalczyk, D.; Biendl, M. Physicochemical and antioxidant properties of biopolymer/candelilla wax emulsion films containing hop extract— A comparative study. Food Hydrocoll. 2016, 60, 384–392. [Google Scholar] [CrossRef]
- Liu, J.; Liu, F.; Ren, T.; Wang, J.; Yang, M.; Yao, Y.; Chen, H. Fabrication of fish gelatin/sodium alginate double network gels for encapsulation of probiotics. J. Sci. Food Agric. 2021, 101, 4398–4408. [Google Scholar] [CrossRef]
- Pan, L.; Li, P.; Tao, Y. Preparation and Properties of Microcrystalline Cellulose/Fish Gelatin Composite Film. Materials 2020, 13, 4370. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Chen, H.; Qiao, P.; Liu, Z. Development and Properties of Fish Gelatin/Oxidized Starch Double Network Film Catalyzed by Thermal Treatment and Schiff’ Base Reaction. Polymers 2019, 11, 2065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Yi, J.; Yu, X.; Wang, Z.; Wang, L. Preparation and characterization of pullulan derivative antibacterial composite films. Mater. Sci. Eng. C 2020, 110, 110721. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.H.; Wang, J.; Wang, D.G.; Zhai, J.Q.; Lu, G.X.; Chen, C.Z. Influence of TiO2 nanoparticles on the performance and inner structure of zein/eugenol films. Food Packag. Shelf Life 2022, 31, 100782. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, L.; Wang, Y.; Chen, Z.; Zhang, M.; Chen, H. Characterization and functional properties of a pectin/tara gum based edible film with ellagitannins from the unripe fruits of Rubus chingii Hu. Food Chem. 2020, 325, 126964. [Google Scholar] [CrossRef]
- Giménez, B.; López De Lacey, A.; Pérez-Santín, E.; López-Caballero, M.E.; Montero, P. Release of active compounds from agar and agar–gelatin films with green tea extract. Food Hydrocoll. 2013, 30, 264–271. [Google Scholar] [CrossRef]
- Iliut, M.; Iosin, M.; Astilean, S. Monitoring the effeces of ultraviolet and visible light on Rb and vitamin A in milk. Environ. Eng. Manag. J. 2013, 12, 2443–2448. [Google Scholar] [CrossRef]
- Aguilar, K.; Garvín, A.; Lara-Sagahón, A.V.; Ibarz, A. Ascorbic acid degradation in aqueous solution during UV-Vis irradiation. Food Chem. 2019, 297, 124864. [Google Scholar] [CrossRef]
- Guneser, O.; Karagul Yuceer, Y. Effect of ultraviolet light on water- and fat-soluble vitamins in cow and goat milk. J. Dairy Sci. 2012, 95, 6230–6241. [Google Scholar] [CrossRef] [Green Version]
- Huang Chidu, C.J.H.X. Kinetics for photo-degradation of chlorophyll in postharvest fruit and vegetable. Trans. CSAE 2008, 10, 233–238. [Google Scholar]
- Raj, S.; Kuniyil, A.M.; Sreenikethanam, A.; Gugulothu, P.; Jeyakumar, R.B.; Bajhaiya, A.K. Microalgae as a Source of Mycosporine-like Amino Acids (MAAs); Advances and Future Prospects. Int. J. Environ. Res. Public Health 2021, 18, 12402. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.C.; Wang, K.; Hao, F.H.; Shang, J.L.; Tang, H.R.; Qiu, B.S. New types ofATP-grasp ligase are associated with the novel pathway for complicated mycosporine-like amino acid production in desiccation-tolerant cyanobacteria. Environ. Microbiol. 2021, 23, 6420–6432. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, R.P.; Sinha, R.P. Biotechnological and industrial significance of cyanobacterial secondary metabolites. Biotechnol. Adv. 2009, 27, 521–539. [Google Scholar] [CrossRef]
- Rosic, N. Mycosporine-Like Amino Acids: Making the Foundation for Organic Personalised Sunscreens. Mar. Drugs 2019, 17, 638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suh, S.; Hwang, J.; Park, M.; Seo, H.; Kim, H.; Lee, J.; Moh, S.; Lee, T. Anti-Inflammation Activities of Mycosporine-Like Amino Acids (MAAs) in Response to UV Radiation Suggest Potential Anti-Skin Aging Activity. Mar. Drugs 2014, 12, 5174–5187. [Google Scholar] [CrossRef] [Green Version]
- Hana, S.S.I.Y.; Mostefa, M.V.M.M. Structural, physicochemical and antioxidant properties of sodium alginate isolated from a Tunisian brown seaweed. Int. J. Biol. Macromol. 2014, 2, 5174–5187. [Google Scholar]
- Kim, D.; Lee, K.W.; Lee, H.J.; Lee, C.Y. Vitamin C Equivalent Antioxidant Capacity (VCEAC) of Phenolic Phytochemicals. J. Agric. Food Chem. 2002, 50, 3713–3717. [Google Scholar] [CrossRef]
- Dou, L.; Li, B.; Zhang, K.; Chu, X.; Hou, H. Physical properties and antioxidant activity of gelatin-sodium alginate edible films with tea polyphenols. Int. J. Biol. Macromol. 2018, 118, 1377–1383. [Google Scholar] [CrossRef]
- Wu, H.; Lei, Y.; Zhu, R.; Zhao, M.; Lu, J.; Xiao, D.; Jiao, C.; Zhang, Z.; Shen, G.; Li, S. Preparation and characterization of bioactive edible packaging films based on pomelo peel flours incorporating tea polyphenol. Food Hydrocoll. 2019, 90, 41–49. [Google Scholar] [CrossRef]
- Jamróz, E.; Kopel, P.; Juszczak, L.; Kawecka, A.; Bytesnikova, Z.; Milosavljević, V.; Kucharek, M.; Makarewicz, M.; Adam, V. Development and characterisation of furcellaran-gelatin films containing SeNPs and AgNPs that have antimicrobial activity. Food Hydrocoll. 2018, 83, 9–16. [Google Scholar] [CrossRef]
- Chandra, M.V.; Shamasundar, B.A. Rheological properties of gelatin prepared from the swim bladders of freshwater fish Catla catla. Food Hydrocoll. 2015, 48, 47–54. [Google Scholar] [CrossRef]
- Abdelhedi, O.; Nasri, R.; Jridi, M.; Kchaou, H.; Nasreddine, B.; Karbowiak, T.; Debeaufort, F.; Nasri, M. Composite bioactive films based on smooth-hound viscera proteins and gelatin: Physicochemical characterization and antioxidant properties. Food Hydrocoll. 2018, 74, 176–186. [Google Scholar] [CrossRef]
- Kageyama, H.; Waditee-Sirisattha, R. Antioxidative, Anti-Inflammatory, and Anti-Aging Properties of Mycosporine-Like Amino Acids: Molecular and Cellular Mechanisms in the Protection of Skin-Aging. Mar. Drugs 2019, 17, 222. [Google Scholar] [CrossRef] [Green Version]
- Silva, R.S.G.; Bandeira, S.F.; Pinto, L.A.A. Characteristics and chemical composition of skins gelatin from cobia (Rachycentron canadum). LWT Food Sci. Technol. 2014, 57, 580–585. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Zhang, N.; Zhou, J.; Dong, S.; Zhang, X.; Guo, L.; Guo, G. Distribution, Contents, and Types of Mycosporine-Like Amino Acids (MAAs) in Marine Macroalgae and a Database for MAAs Based on These Characteristics. Mar. Drugs 2020, 18, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kchaou, H.; Benbettaïeb, N.; Jridi, M.; Abdelhedi, O.; Karbowiak, T.; Brachais, C.; Léonard, M.; Debeaufort, F.; Nasri, M. Enhancement of structural, functional and antioxidant properties of fish gelatin films using Maillard reactions. Food Hydrocoll. 2018, 83, 326–339. [Google Scholar] [CrossRef]
- Tan, W.; Zhang, J.; Zhao, X.; Li, Q.; Dong, F.; Guo, Z. Preparation and physicochemical properties of antioxidant chitosan ascorbate/methylcellulose composite films. Int. J. Biol. Macromol. 2020, 146, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Zijing, D.Y.C.H. Research on the Preparation and Properties of the Sodium Alginate-Fish Gelatin Composite Edible Film. J. Chin. Inst. Food Sci. Technol. 2020, 10, 134–140. [Google Scholar]
- Tan, J.B.L.; Lim, Y.Y. Critical analysis of current methods for assessing the in vitro antioxidant and antibacterial activity of plant extracts. Food Chem. 2015, 172, 814–822. [Google Scholar] [CrossRef]
- Li, J.; Miao, J.; Wu, J.; Chen, S.; Zhang, Q. Preparation and characterization of active gelatin-based films incorporated with natural antioxidants. Food Hydrocoll. 2014, 37, 166–173. [Google Scholar] [CrossRef]
- Lin, D.; Zheng, Y.; Wang, X.; Huang, Y.; Ni, L.; Chen, X.; Wu, Z.; Huang, C.; Yi, Q.; Li, J.; et al. Study on physicochemical properties, antioxidant and antimicrobial activity of okara soluble dietary fiber/sodium carboxymethyl cellulose/thyme essential oil active edible composite films incorporated with pectin. Int. J. Biol. Macromol. 2020, 165, 1241–1249. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Estaca, J.; Giménez, B.; Montero, P.; Gómez-Guillén, M.C. Incorporation of antioxidant borage extract into edible films based on sole skin gelatin or a commercial fish gelatin. J. Food Eng. 2009, 92, 78–85. [Google Scholar] [CrossRef]
- Pereda, M.; Dufresne, A.; Aranguren, M.I.; Marcovich, N.E. Polyelectrolyte films based on chitosan/olive oil and reinforced with cellulose nanocrystals. Carbohyd. Polym. 2014, 101, 1018–1026. [Google Scholar] [CrossRef] [PubMed]








| Samples | L | a | b | ΔE |
|---|---|---|---|---|
| FSM-0 | 74.21 ± 1.79 a | 0.25 ± 0.17 c | 0.73 ± 0.19 d | 0.00 |
| FSM-1.25 | 68.24 ± 0.77 b | 0.29 ± 0.12 c | 2.22 ± 0.37 c | 6.15 |
| FSM-2.5 | 67.96 ± 1.97 b | 0.36 ± 0.18 bc | 2.30 ± 0.11 c | 6.45 |
| FSM-3.75 | 67.32 ± 3.78 b | 0.62 ± 0.07 b | 3.70 ± 0.28 b | 7.51 |
| FSM-5 | 64.76 ± 5.23 b | 1.30 ± 0.15 a | 6.03 ± 0.50 a | 10.89 |
| Samples | Water Content(%) | Solubility(%) | Swelling Ability | WVP(g·mm/m2·h·kPa) |
|---|---|---|---|---|
| FSM-0 | 7.01 ± 0.43 a | 24.3 6± 5.25 b | 23.65 ± 3.40 a | 0.01742 ± 0.00047 a |
| FSM-1.25 | 7.18 ± 0.40 a | 30.50 ± 1.83 ab | 25.13 ± 11.41 a | 0.01575 ± 0.00119 a |
| FSM-2.5 | 6.05 ± 0.91 a | 27.73 ± 5.37 ab | 23.81 ± 4.09 a | 0.01780 ± 0.00249 a |
| FSM-3.75 | 6.44 ± 0.93 a | 29.79 ± 0.51 ab | 29.26 ± 5.60 a | 0.01733 ± 0.00348 a |
| FSM-5 | 6.35 ± 0.77 a | 32.90 ± 1.42 a | 23.12 ± 8.62 a | 0.01760 ± 0.00156 a |
| Samples | Light Transmission (%) of Samples at Different Wavelengths (nm) | |||||
|---|---|---|---|---|---|---|
| 200 | 280 | 350 | 400 | 600 | 800 | |
| FSM-0 | 0.70 | 15.10 | 74.30 | 80.80 | 85.30 | 86.50 |
| FSM-1.25 | 0.50 | 6.70 | 2.30 | 75.70 | 84.30 | 86.10 |
| FSM-2.5 | 0.60 | 5.85 | 1.45 | 72.60 | 85.15 | 87.25 |
| FSM-3.75 | 0.40 | 4.28 | 1.38 | 71.15 | 84.83 | 86.68 |
| FSM-5 | 0.30 | 5.00 | 0.60 | 69.50 | 85.80 | 88.40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gan, J.; Guan, C.; Zhang, X.; Sun, L.; Zhang, Q.; Pan, S.; Zhang, Q.; Chen, H. The Preparation of Anti-Ultraviolet Composite Films Based on Fish Gelatin and Sodium Alginate Incorporated with Mycosporine-like Amino Acids. Polymers 2022, 14, 2980. https://doi.org/10.3390/polym14152980
Gan J, Guan C, Zhang X, Sun L, Zhang Q, Pan S, Zhang Q, Chen H. The Preparation of Anti-Ultraviolet Composite Films Based on Fish Gelatin and Sodium Alginate Incorporated with Mycosporine-like Amino Acids. Polymers. 2022; 14(15):2980. https://doi.org/10.3390/polym14152980
Chicago/Turabian StyleGan, Jing, Chenxia Guan, Xiaoyu Zhang, Lirong Sun, Qinling Zhang, Shihui Pan, Qian Zhang, and Hao Chen. 2022. "The Preparation of Anti-Ultraviolet Composite Films Based on Fish Gelatin and Sodium Alginate Incorporated with Mycosporine-like Amino Acids" Polymers 14, no. 15: 2980. https://doi.org/10.3390/polym14152980
APA StyleGan, J., Guan, C., Zhang, X., Sun, L., Zhang, Q., Pan, S., Zhang, Q., & Chen, H. (2022). The Preparation of Anti-Ultraviolet Composite Films Based on Fish Gelatin and Sodium Alginate Incorporated with Mycosporine-like Amino Acids. Polymers, 14(15), 2980. https://doi.org/10.3390/polym14152980