A Diluted Electrolyte for Long-Life Sulfurized Polyacrylonitrile-Based Anode-Free Li-S Batteries
Abstract
:1. Introduction
2. Experimental
2.1. Synthesis of Materials
2.1.1. Materials
2.1.2. Preparation of pPAN/SeS2
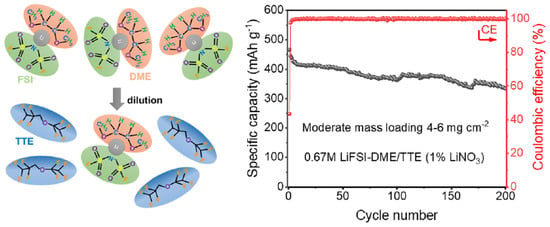
2.1.3. Diluted Electrolyte Configuration
2.2. Characterizations
2.3. Electrochemical Measurements
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, M.; Lu, J.; Chen, Z.; Amine, K. 30 years of lithium-ion batteries. Adv. Mater. 2018, 30, 1800561. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Y.; Wu, X.; Wang, K.; Feng, Z.; Cheng, T.; Liu, Y.; Wang, M.; Chen, R.; Xu, L.; Zhou, J. An overview on the advances of LiCoO2 cathodes for lithium-ion batteries. Adv. Energy Mater. 2021, 11, 2000982. [Google Scholar] [CrossRef]
- Li, J.; Ma, Z.-F. Past and present of LiFePO4: From fundamental research to industrial applications. Chem 2019, 5, 3–6. [Google Scholar] [CrossRef]
- de Biasi, L.; Schwarz, B.; Brezesinski, T.; Hartmann, P.; Janek, J.; Ehrenberg, H. Chemical, structural, and electronic aspects of formation and degradation behavior on different length scales of Ni-rich NCM and Li-rich HE-NCM cathode materials in Li-ion batteries. Adv. Mater. 2019, 31, 1900985. [Google Scholar] [CrossRef]
- Fan, L.; Ma, R.; Zhang, Q.; Jia, X.; Lu, B. Graphite anode for a potassium-ion battery with unprecedented performance. Angew. Chem. Int. Ed. 2019, 131, 10610–10615. [Google Scholar] [CrossRef]
- Cheng, X.-B.; Zhang, R.; Zhao, C.-Z.; Zhang, Q. Toward safe lithium metal anode in rechargeable batteries: A review. Chem. Rev. 2017, 117, 10403–10473. [Google Scholar] [CrossRef]
- Wu, J.; Lin, C.; Liang, Q.; Zhou, G.; Liu, J.; Liang, G.; Wang, M.; Li, B.; Hu, L.; Ciucci, F.; et al. Sodium-rich NASICON-structured cathodes for boosting the energy density and lifespan of sodium-free-anode sodium metal batteries. InfoMat 2022, 4, e12288. [Google Scholar] [CrossRef]
- Fang, C.; Li, J.; Zhang, M.; Zhang, Y.; Yang, F.; Lee, J.Z.; Lee, M.-H.; Alvarado, J.; Schroeder, M.A.; Yang, Y. Quantifying inactive lithium in lithium metal batteries. Nature 2019, 572, 511–515. [Google Scholar] [CrossRef]
- Tarascon, J.-M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. In Materials for Sustainable Energy: A Collection of Peer-Reviewed Research and Review Articles from Nature Publishing Group; CO-PUBLISHED WITH World Scientific Publishing Comp: Singapore, 2011; pp. 171–179. [Google Scholar]
- Liu, J.; Bao, Z.; Cui, Y.; Dufek, E.J.; Goodenough, J.B.; Khalifah, P.; Li, Q.; Liaw, B.Y.; Liu, P.; Manthiram, A. Pathways for practical high-energy long-cycling lithium metal batteries. Nat. Energy 2019, 4, 180–186. [Google Scholar] [CrossRef]
- Xia, C.; Kwok, C.; Nazar, L. A high-energy-density lithium-oxygen battery based on a reversible four-electron conversion to lithium oxide. Science 2018, 361, 777–781. [Google Scholar] [CrossRef]
- Fang, R.; Zhao, S.; Sun, Z.; Wang, D.W.; Cheng, H.M.; Li, F. More reliable lithium-sulfur batteries: Status, solutions and prospects. Adv. Mater. 2017, 29, 1606823. [Google Scholar] [CrossRef]
- Yan, K.; Lu, Z.; Lee, H.-W.; Xiong, F.; Hsu, P.-C.; Li, Y.; Zhao, J.; Chu, S.; Cui, Y. Selective deposition and stable encapsulation of lithium through heterogeneous seeded growth. Nat. Energy 2016, 1, 16010. [Google Scholar] [CrossRef]
- Zhang, R.; Cheng, X.B.; Zhao, C.Z.; Peng, H.J.; Shi, J.L.; Huang, J.Q.; Wang, J.; Wei, F.; Zhang, Q. Conductive nanostructured scaffolds render low local current density to inhibit lithium dendrite growth. Adv. Mater. 2016, 28, 2155–2162. [Google Scholar] [CrossRef]
- Zhang, R.; Li, N.W.; Cheng, X.B.; Yin, Y.X.; Zhang, Q.; Guo, Y.G. Advanced micro/nanostructures for lithium metal anodes. Adv. Sci. 2017, 4, 1600445. [Google Scholar] [CrossRef]
- Lorandi, F.; Liu, T.; Fantin, M.; Manser, J.; Al-Obeidi, A.; Zimmerman, M.; Matyjaszewski, K.; Whitacre, J.F. Comparative performance of ex situ artificial solid electrolyte interphases for Li metal batteries with liquid electrolytes. iScience 2021, 24, 102578. [Google Scholar] [CrossRef]
- Lin, D.; Liu, Y.; Liang, Z.; Lee, H.-W.; Sun, J.; Wang, H.; Yan, K.; Xie, J.; Cui, Y. Layered reduced graphene oxide with nanoscale interlayer gaps as a stable host for lithium metal anodes. Nat. Nanotechnol. 2016, 11, 626–632. [Google Scholar] [CrossRef]
- Pu, J.; Li, J.; Zhang, K.; Zhang, T.; Li, C.; Ma, H.; Zhu, J.; Braun, P.V.; Lu, J.; Zhang, H. Conductivity and lithiophilicity gradients guide lithium deposition to mitigate short circuits. Nat. Commun. 2019, 10, 1896. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, J.; Lei, L.; Li, P.; Chen, J.; Zhang, Y.; Wang, Y.; Ma, Y.; Wang, D. Dynamic intelligent Cu current collectors for ultrastable lithium metal anodes. Nano Lett. 2020, 20, 3403–3410. [Google Scholar] [CrossRef]
- Zheng, H.; Zhang, Q.; Chen, Q.; Xu, W.; Xie, Q.; Cai, Y.; Ma, Y.; Qiao, Z.; Luo, Q.; Lin, J. 3D lithiophilic–lithiophobic–lithiophilic dual-gradient porous skeleton for highly stable lithium metal anode. J. Mater. Chem. A. 2020, 8, 313–322. [Google Scholar] [CrossRef]
- Ren, X.; Zou, L.; Jiao, S.; Mei, D.; Engelhard, M.H.; Li, Q.; Lee, H.; Niu, C.; Adams, B.D.; Wang, C. High-concentration ether electrolytes for stable high-voltage lithium metal batteries. ACS Energy Lett. 2019, 4, 896–902. [Google Scholar] [CrossRef]
- Jiao, S.; Ren, X.; Cao, R.; Engelhard, M.H.; Liu, Y.; Hu, D.; Mei, D.; Zheng, J.; Zhao, W.; Li, Q. Stable cycling of high-voltage lithium metal batteries in ether electrolytes. Nat. Energy 2018, 3, 739–746. [Google Scholar] [CrossRef]
- Liu, W.; Liu, P.; Mitlin, D. Review of emerging concepts in SEI analysis and artificial SEI membranes for lithium, sodium, and potassium metal battery anodes. Adv. Energy Mater. 2020, 10, 2002297. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, R.; Zhang, D.; Liu, J.; Liu, F.; Cui, J.; Lin, Z.; Wu, J.; Zhu, M. Constructing Li-Rich artificial SEI layer in alloy–polymer composite electrolyte to achieve high ionic conductivity for all-solid-state lithium metal batteries. Adv. Mater. 2021, 33, 2004711. [Google Scholar] [CrossRef]
- Zhu, J.; Li, P.; Chen, X.; Legut, D.; Fan, Y.; Zhang, R.; Lu, Y.; Cheng, X.; Zhang, Q. Rational design of graphitic-inorganic Bi-layer artificial SEI for stable lithium metal anode. Energy Storage Mater. 2019, 16, 426–433. [Google Scholar] [CrossRef]
- Tan, J.; Matz, J.; Dong, P.; Shen, J.; Ye, M. A growing appreciation for the role of LiF in the solid electrolyte interphase. Adv. Energy Mater. 2021, 11, 2100046. [Google Scholar] [CrossRef]
- Wood, K.N.; Steirer, K.X.; Hafner, S.E.; Ban, C.; Santhanagopalan, S.; Lee, S.-H.; Teeter, G. Operando X-ray photoelectron spectroscopy of solid electrolyte interphase formation and evolution in Li2S-P2S5 solid-state electrolytes. Nat. Commun. 2018, 9, 2490. [Google Scholar] [CrossRef] [PubMed]
- Hope, M.A.; Rinkel, B.L.; Gunnarsdóttir, A.B.; Märker, K.; Menkin, S.; Paul, S.; Sergeyev, I.V.; Grey, C.P. Selective NMR observation of the SEI–metal interface by dynamic nuclear polarisation from lithium metal. Nat. Commun. 2020, 11, 2224. [Google Scholar] [CrossRef]
- Ramasubramanian, A.; Yurkiv, V.; Foroozan, T.; Ragone, M.; Shahbazian-Yassar, R.; Mashayek, F. Stability of Solid-Electrolyte Interphase (SEI) on the Lithium Metal Surface in Lithium Metal Batteries (LMBs). ACS Appl. Energ. Mater. 2020, 3, 10560–10567. [Google Scholar] [CrossRef]
- Wu, J.; Ihsan-Ul-Haq, M.; Chen, Y.; Kim, J.-K. Understanding solid electrolyte interphases: Advanced characterization techniques and theoretical simulations. Nano Energy 2021, 89, 106489. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Li, T.; Li, B.Q.; Zhang, R.; Shi, P.; Yan, C.; Huang, J.Q.; Zhang, Q. A sustainable solid electrolyte interphase for high-energy-density lithium metal batteries under practical conditions. Angew. Chem. Int. Ed. 2020, 132, 3278–3283. [Google Scholar] [CrossRef]
- Tikekar, M.D.; Choudhury, S.; Tu, Z.; Archer, L.A. Design principles for electrolytes and interfaces for stable lithium-metal batteries. Nat. Energy 2016, 1, 16114. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Chen, X.; Cheng, X.B.; Li, B.Q.; Shen, X.; Yan, C.; Huang, J.Q.; Zhang, Q. Highly stable lithium metal batteries enabled by regulating the solvation of lithium ions in nonaqueous electrolytes. Angew. Chem. Int. Ed. 2018, 57, 5301–5305. [Google Scholar] [CrossRef]
- May, R.; Fritzsching, K.J.; Livitz, D.; Denny, S.R.; Marbella, L.E. Rapid interfacial exchange of Li ions dictates high Coulombic efficiency in Li metal anodes. ACS Energy Lett. 2021, 6, 1162–1169. [Google Scholar] [CrossRef]
- Dai, H.; Xi, K.; Liu, X.; Lai, C.; Zhang, S. Cationic surfactant-based electrolyte additives for uniform lithium deposition via lithiophobic repulsion mechanisms. J. Am. Chem. Soc. 2018, 140, 17515–17521. [Google Scholar] [CrossRef]
- Suo, L.; Xue, W.; Gobet, M.; Greenbaum, S.G.; Wang, C.; Chen, Y.; Yang, W.; Li, Y.; Li, J. Fluorine-donating electrolytes enable highly reversible 5-V-class Li metal batteries. Proc. Natl. Acad. Sci. USA 2018, 115, 1156–1161. [Google Scholar] [CrossRef]
- Wang, J.; Yamada, Y.; Sodeyama, K.; Chiang, C.H.; Tateyama, Y.; Yamada, A. Superconcentrated electrolytes for a high-voltage lithium-ion battery. Nat. Commun. 2016, 7, 12032. [Google Scholar] [CrossRef]
- Yu, L.; Chen, S.; Lee, H.; Zhang, L.; Engelhard, M.H.; Li, Q.; Jiao, S.; Liu, J.; Xu, W.; Zhang, J.-G. A localized high-concentration electrolyte with optimized solvents and lithium difluoro (oxalate) borate additive for stable lithium metal batteries. ACS Energy Lett. 2018, 3, 2059–2067. [Google Scholar] [CrossRef]
- Cao, X.; Zou, L.; Matthews, B.E.; Zhang, L.; He, X.; Ren, X.; Engelhard, M.H.; Burton, S.D.; El-Khoury, P.Z.; Lim, H.-S. Optimization of fluorinated orthoformate based electrolytes for practical high-voltage lithium metal batteries. Energy Storage Mater. 2021, 34, 76–84. [Google Scholar] [CrossRef]
- Ren, X.; Zhang, X.; Shadike, Z.; Zou, L.; Jia, H.; Cao, X.; Engelhard, M.H.; Matthews, B.E.; Wang, C.; Arey, B.W. Designing advanced in situ electrode/electrolyte interphases for wide temperature operation of 4.5 V Li||LiCoO2 batteries. Adv. Mater. 2020, 32, 2004898. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Cheng, X.B.; Chen, X.; Yan, C.; Zhang, Q. Fluoroethylene carbonate additives to render uniform Li deposits in lithium metal batteries. Adv. Funct. Mater. 2017, 27, 1605989. [Google Scholar] [CrossRef]
- Qian, J.; Henderson, W.A.; Xu, W.; Bhattacharya, P.; Engelhard, M.; Borodin, O.; Zhang, J.-G. High rate and stable cycling of lithium metal anode. Nat. Commun. 2015, 6, 6362. [Google Scholar] [CrossRef]
- Zheng, J.; Chen, S.; Zhao, W.; Song, J.; Engelhard, M.H.; Zhang, J.-G. Extremely stable sodium metal batteries enabled by localized high-concentration electrolytes. ACS Energy Lett. 2018, 3, 315–321. [Google Scholar] [CrossRef]
- Chen, S.; Zheng, J.; Yu, L.; Ren, X.; Engelhard, M.H.; Niu, C.; Lee, H.; Xu, W.; Xiao, J.; Liu, J. High-efficiency lithium metal batteries with fire-retardant electrolytes. Joule 2018, 2, 1548–1558. [Google Scholar] [CrossRef]
- Seh, Z.W.; Sun, Y.; Zhang, Q.; Cui, Y. Designing high-energy lithium–sulfur batteries. Chem. Soc. Rev. 2016, 45, 5605–5634. [Google Scholar] [CrossRef]
- Li, G.; Wang, S.; Zhang, Y.; Li, M.; Chen, Z.; Lu, J. Revisiting the role of polysulfides in lithium–sulfur batteries. Adv. Mater. 2018, 30, 1705590. [Google Scholar] [CrossRef]
- Ji, X.; Lee, K.T.; Nazar, L.F. A highly ordered nanostructured carbon–sulphur cathode for lithium–sulphur batteries. Nat. Mater. 2009, 8, 500–506. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, J.; Lu, Y.; Lou, X.W. A pyrolyzed polyacrylonitrile/selenium disulfide composite cathode with remarkable lithium and sodium storage performances. Sci. Adv. 2018, 4, eaat1687. [Google Scholar] [CrossRef]
- Luo, C.; Zhu, Y.; Wen, Y.; Wang, J.; Wang, C. Carbonized polyacrylonitrile-stabilized SeSx cathodes for long cycle life and high power density lithium ion batteries. Adv. Funct. Mater. 2014, 24, 4082–4089. [Google Scholar] [CrossRef]
- Zhang, W.; Li, S.; Wang, L.; Wang, X.; Xie, J. Insight into sulfur-rich selenium sulfide/pyrolyzed polyacrylonitrile cathodes for Li–S batteries. Sustain. Energy Fuels 2020, 4, 3588–3596. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, H.; Zhang, N.; Liu, H.; Chen, Z.; Zhang, L.; Guo, S.; Li, D.; Xu, J. One-step in situ preparation of polymeric selenium sulfide composite as a cathode material for enhanced sodium/potassium storage. ACS. Appl. Mater. Inter. 2019, 11, 29807–29813. [Google Scholar] [CrossRef]
- Wu, H.; Jia, H.; Wang, C.; Zhang, J.G.; Xu, W. Recent progress in understanding solid electrolyte interphase on lithium metal anodes. Adv. Energy Mater. 2021, 11, 2003092. [Google Scholar] [CrossRef]
- Kang, D.W.; Moon, J.; Choi, H.-Y.; Shin, H.-C.; Kim, B.G. Stable cycling and uniform lithium deposition in anode-free lithium-metal batteries enabled by a high-concentration dual-salt electrolyte with high LiNO3 content. J. Power Sources 2021, 490, 229504. [Google Scholar] [CrossRef]
- Fu, J.; Ji, X.; Chen, J.; Chen, L.; Fan, X.; Mu, D.; Wang, C. Lithium nitrate regulated sulfone electrolytes for lithium metal batteries. Angew. Chem. Int. Ed. 2020, 132, 22378–22385. [Google Scholar] [CrossRef]
- Lin, S.; Zhao, J. Functional electrolyte of fluorinated ether and ester for stabilizing both 4.5 V LiCoO2 cathode and lithium metal anode. ACS. Appl. Mater. Inter. 2020, 12, 8316–8323. [Google Scholar] [CrossRef]
- Fan, X.; Chen, L.; Ji, X.; Deng, T.; Hou, S.; Chen, J.; Zheng, J.; Wang, F.; Jiang, J.; Xu, K. Highly fluorinated interphases enable high-voltage Li-metal batteries. Chem 2018, 4, 174–185. [Google Scholar] [CrossRef]
- Jung, Y.; Park, S.; Kim, J.K.; Kim, M.; Kang, B. Toward achieving high kinetics in anodeless Li2S battery: Surface modification of Cu current collector. Adv. Funct. Mater. 2022, 32, 2109759. [Google Scholar] [CrossRef]
- Nanda, S.; Gupta, A.; Manthiram, A. A lithium–sulfur cell based on reversible lithium deposition from a Li2S cathode host onto a hostless-anode substrate. Adv. Energy Mater. 2018, 8, 1801556. [Google Scholar] [CrossRef]
- Chen, J.; Xiang, J.; Chen, X.; Yuan, L.; Li, Z.; Huang, Y. Li2S-based anode-free full batteries with modified Cu current collector. Energy Storage Mater. 2020, 30, 179–186. [Google Scholar] [CrossRef]




| Electrolyte | Concentration | Li Plating/Stripping CE | Ref. |
|---|---|---|---|
| LiFSI-DME/TTE | 0.67 M | 99.3%, 200 cycles,0.5 mA cm−2, 1 mAh cm−2 | This work |
| LiFSI-DME | 4 M | 98.5%, 300 cycles, 1 mA cm−2, 1 mAh cm−2 | [42] |
| LiNO3 + LiFSI-DME | 4 M | 98.5%, 400 cycles, 0.5 mA cm−2, 0.5 mAh cm−2 | [53] |
| LiTFSI-SL | 1.3 M | 96.6%, 100 cycles, 0.5 mA cm−2, 1 mAh cm−2 | [54] |
| LiPF6 + LiDFOB- FEC/DMC/HFE | 1.2 M | 98%, 200 cycles, 1 mA cm−2, 1 mAh cm−2 | [55] |
| LiFSI-DMC | 10 M | 99.2%, 200 cycles, 0.2 mA cm−2, 2.5 mAh cm−2 | [56] |
| Battery Chemistry | Cycling Stability | Loading | Ref. |
|---|---|---|---|
| Cu||pPAN/SeS2 | 71.5% retention over 200 cycles at 0.1 A g−1 | 4–6 mg cm−2 | This work |
| ATCu||Li2S | 64.8% retention over 120 cycles at 1.166 A g−1 | 4.5 mg cm−2 | [57] |
| Cu||Li2S | 70% retention over 100 cycles at 0.1166 A g−1 | 4 mg cm−2 | [58] |
| Au/Cu||Li2S | 69.5% retention over 150 cycles at 0.1 C | 4 mg cm−2 | [59] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, T.; Ren, X.; Hu, L.; Teng, W.; Wang, X.; Wu, G.; Liu, J.; Nan, D.; Li, B.; Yu, X. A Diluted Electrolyte for Long-Life Sulfurized Polyacrylonitrile-Based Anode-Free Li-S Batteries. Polymers 2022, 14, 3312. https://doi.org/10.3390/polym14163312
Ma T, Ren X, Hu L, Teng W, Wang X, Wu G, Liu J, Nan D, Li B, Yu X. A Diluted Electrolyte for Long-Life Sulfurized Polyacrylonitrile-Based Anode-Free Li-S Batteries. Polymers. 2022; 14(16):3312. https://doi.org/10.3390/polym14163312
Chicago/Turabian StyleMa, Ting, Xiuyun Ren, Liang Hu, Wanming Teng, Xiaohu Wang, Guanglei Wu, Jun Liu, Ding Nan, Baohua Li, and Xiaoliang Yu. 2022. "A Diluted Electrolyte for Long-Life Sulfurized Polyacrylonitrile-Based Anode-Free Li-S Batteries" Polymers 14, no. 16: 3312. https://doi.org/10.3390/polym14163312
APA StyleMa, T., Ren, X., Hu, L., Teng, W., Wang, X., Wu, G., Liu, J., Nan, D., Li, B., & Yu, X. (2022). A Diluted Electrolyte for Long-Life Sulfurized Polyacrylonitrile-Based Anode-Free Li-S Batteries. Polymers, 14(16), 3312. https://doi.org/10.3390/polym14163312