Adopting Sustainable Jatropha Oil Bio-Based Polymer Membranes as Alternatives for Environmental Remediation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Filtration Method
2.3. Determination of Zeta Potential at Different Solution pH Value
2.4. Parametric Studies by Single Factor Experiment
2.5. Response Surface Methodology
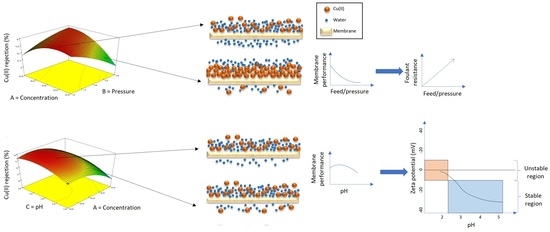
3. Results and Discussion
3.1. Parametric Studies by Single-Factor Experiments
3.1.1. Effect of Cu(II) ions Feed Concentration
3.1.2. Effect of Feed Pressure
3.1.3. Effect of Solution pH Value
3.2. Optimization of Cu(II) ions Filtration by Response Surface Methodology (RSM)
3.2.1. Model Fitting
3.2.2. Analysis of Variance (ANOVA)
3.2.3. Residual Analysis
3.2.4. Effect of Filtration Factors Based on the Response Surface Plots Analysis
3.2.5. Optimization and Model Validation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ramakrishnaiah, C.R.; Sadashivaiah, C.; Ranganna, G. Assessment of Water Quality Index for the Groundwater in Tumkur Taluk, Karnataka State, India. E-J. Chem. 2009, 6, 523–530. [Google Scholar] [CrossRef]
- Rowell, R.M.; Boving, T.B. Water Filtration Using Biomaterials. In Sustainable Development in the Forest Products Industry; UFP Edition: Lisbon, Portugal, 2010; pp. 213–252. [Google Scholar]
- Nordstrom, D.K.; Alpers, C.N.; Ptacek, C.J.; Blowes, D.W. Negative PH and Extremely Acidic Mine Waters from Iron Mountain, California. Environ. Sci. Technol. 2000, 34, 254–258. [Google Scholar] [CrossRef]
- Ilhan, F.; Ulucan-Altuntas, K.; Avsar, Y.; Kurt, U.; Saral, A. Electrocoagulation Process for the Treatment of Metal-Plating Wastewater: Kinetic Modeling and Energy Consumption. Front. Environ. Sci. Eng. 2019, 13, 73. [Google Scholar] [CrossRef]
- Mustapha, M.U. Removal of Heavy Metals from Electroplating Wastewater Using Bacteria. Master’s Thesis, Universiti Putra Malaysia, Serdang, Selangor, 2016. [Google Scholar]
- Jopony, M.; Tongkul, F. Acid Mine Drainages at Mamut Copper Mine, Sabah, Malaysia. Borneo Sci. 2009, 24, 83–94. [Google Scholar]
- Polluted Runoff: Nonpoint Source (NPS) Pollution. Available online: https://www.epa.gov/nps/abandoned-mine-drainage (accessed on 1 July 2022).
- Ruijing, S.; Liang, B.; Guan, J. Leaching Effects of Metal from Electroplating Sludge under Phosphate Participation in Hydrochloric Acid Medium. Procedia Environ. Sci. 2016, 31, 361–365. [Google Scholar]
- Mansfeldt, T.; Dohrmann, R. Chemical and Mineralogical Characterization of Blast-Furnace Sludge from an Abandoned Landfill. Environ. Sci. Technol. 2004, 38, 5977–5984. [Google Scholar] [CrossRef] [PubMed]
- Jordán, M.M.; Almendro-Candel, M.B.; Romero, M.; Rincón, J.M. Application of Sewage Sludge in the Manufacturing of Ceramic Tile Bodies. Appl. Clay Sci. 2005, 30, 219–224. [Google Scholar] [CrossRef]
- Boving, T.B. Chemical Retention Capacity of a Newly Constructed Roadway Runoff Detention Pond System. In URI Transportation Center: Research Report; University of Rhode Island Transportation Center: Kingston, RI, USA, 2002. [Google Scholar]
- Göbel, P.; Dierkes, C.; Coldwey, W.G. Storm Water Runoff Concentration Matrix for Urban Areas. J. Cont. Hydrol. 2007, 91, 26–42. [Google Scholar] [CrossRef]
- Hoffman, E.J.; Mills, E.J.; Latimer, J.S.; Quinn, J.G. Urban Runoff as a Source of Polycyclic Aromatic Hydrocarbons to Coastal Waters. Environ. Sci. Technol. 1984, 18, 580–587. [Google Scholar] [CrossRef]
- Aji, M.P.; Wiguna, P.A.; Karunawan, J.; Wati, A.L. Sulhadi Removal of Heavy Metal Nickel-Ions from Wastewaters Using Carbon Nanodots from Frying Oil. Procedia Eng. 2017, 170, 36–40. [Google Scholar] [CrossRef]
- Prabhu, P.P.; Prabhu, B. A Review on Removal of Heavy Metal Ions from Wastewater Using Natural/ Modified Bentonite. MATEC Web Conf. 2018, 144, 02021. [Google Scholar] [CrossRef]
- Ahmad, K.; Shah, H.-R.; Ahmad, M.; Ahmed, M.; Naseem, K.; Riaz, N.; Muhammad, A.; Ayub, A.; Ahmad, M.; Ahmad, Z.; et al. Comparative Study Between Two Zeolitic Imidazolate Frameworks as Adsorbents for Removal of Organoarsenic, As(III) and As(V) Species from Water. Braz. J. Anal. Chem. 2022, 9, 78–97. [Google Scholar] [CrossRef]
- Aljohny, B.O.; Ahmad, Z.; Shah, S.A.; Anwar, Y.; Khan, S.A. Cellulose Acetate Composite Films Fabricated with Zero-valent Iron Nanoparticles and Its Use in the Degradation of Persistent Organic Pollutants. Appl. Organomet. Chem. 2020, 34, e5892. [Google Scholar] [CrossRef]
- Yao, Q.; Fan, B.; Xiong, Y.; Jin, C.; Sun, Q.; Sheng, C. 3D Assembly Based on 2D Structure of Cellulose Nanofibril/Graphene Oxide Hybrid Aerogel for Adsorptive Removal of Antibiotics in Water. Sci. Rep. 2017, 7, 45914. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, K.; Shah, H.-U.-R.; Ashfaq, M.; Nawaz, H. Removal of Decidedly Lethal Metal Arsenic from Water Using Metal Organic Frameworks: A Critical Review. Rev. Inorg. Chem. 2022, 42, 197–227. [Google Scholar] [CrossRef]
- Kapoor, A.; Balasubramanian, S.; Kavitha, E.; Poonguzhali, E.; Prabhakar, S. Role of Membranes in Wastewater Treatment. In Water Pollution and Remediation: Photocatalysis; Inamuddin, Ahamed, M.I., Lichtfouse, E., Eds.; Environmental Chemistry for a Sustainable World; Springer International Publishing: Cham, Switzerland, 2021; Volume 57, pp. 247–281. ISBN 978-3-030-54722-6. [Google Scholar]
- Mulder, M. Basic Principles of Membrane Technology, 2nd ed.; Kluwer Academic: Dordrecht, The Netherlands, 1996; ISBN 978-0-7923-4247-2. [Google Scholar]
- Ruhal, R.; Choudhury, B. Membrane Separation and Design. In Handbook of Food Process Design; Ahmed, J., Rahman, M.S., Eds.; Wiley-Blackwell: Oxford, UK, 2012; pp. 769–788. ISBN 978-1-4443-9827-4. [Google Scholar]
- Strathmann, H. Introduction to Membrane Science and Technology; Bachelor–Master; Wiley-VCH: Weinheim, Germany, 2011; ISBN 978-3-527-32451-4. [Google Scholar]
- Mohshim, D.F.; Mukhtar, H.B.; Man, Z.; Nasir, R. Latest Development on Membrane Fabrication for Natural Gas Purification: A Review. J. Eng. 2013, 2013, 101746. [Google Scholar] [CrossRef]
- Baker, R.W. Membrane Technology and Applications, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2012; ISBN 978-1-118-35971-6. [Google Scholar]
- Ulbricht, M. Advanced Functional Polymer Membranes. Polymer 2006, 47, 2217–2262. [Google Scholar] [CrossRef]
- Peyravi, M.; Rahimpour, A.; Jahanshahi, M. Thin Film Composite Membranes with Modified Polysulfone Supports for Organic Solvent Nanofiltration. J. Membr. Sci. 2012, 423–424, 225–237. [Google Scholar] [CrossRef]
- Ibrahim, S.; Riahi, O.; Said, S.M.; Sabri, M.F.M.; Rozali, S. Biopolymers From Crop Plants. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2019; p. B9780128035818115000. ISBN 978-0-12-803581-8. [Google Scholar]
- Phan, D.-N.; Khan, M.Q.; Nguyen, N.-T.; Phan, T.-T.; Ullah, A.; Khatri, M.; Kien, N.N.; Kim, I.-S. A Review on the Fabrication of Several Carbohydrate Polymers into Nanofibrous Structures Using Electrospinning for Removal of Metal Ions and Dyes. Carbohydr. Polym. 2021, 252, 117175. [Google Scholar] [CrossRef]
- Russo, T.; Fucile, P.; Giacometti, R.; Sannino, F. Sustainable Removal of Contaminants by Biopolymers: A Novel Approach for Wastewater Treatment. Current State and Future Perspectives. Processes 2021, 9, 719. [Google Scholar] [CrossRef]
- Karimi, M.B.; Khanbabaei, G.; Sadeghi, G.M.M. Vegetable Oil-Based Polyurethane Membrane for Gas Separation. J. Membr. Sci. 2017, 527, 198–206. [Google Scholar] [CrossRef]
- Ibrahim, S.; Ahmad, A.; Mohamed, N. Characterization of Novel Castor Oil-Based Polyurethane Polymer Electrolytes. Polymers 2015, 7, 747–759. [Google Scholar] [CrossRef]
- Gowthaman, N.S.K.; Lim, H.N.; Sreeraj, T.R.; Amalraj, A.; Gopi, S. Advantages of Biopolymers over Synthetic Polymers. In Biopolymers and Their Industrial Applications; Elsevier: Amsterdam, The Netherlands, 2021; pp. 351–372. ISBN 978-0-12-819240-5. [Google Scholar]
- Wang, D.; Yuan, H.; Chen, Y.; Ni, Y.; Huang, L.; Mondal, A.K.; Lin, S.; Huang, F.; Zhang, H. A Cellulose-Based Nanofiltration Membrane with a Stable Three-Layer Structure for the Treatment of Drinking Water. Cellulose 2020, 27, 8237–8253. [Google Scholar] [CrossRef]
- Abidin, Z.Z.; Ismail, N.; Yunus, R.; Ahamad, I.S.; Idris, A. A Preliminary Study on Jatropha Curcas as Coagulant in Wastewater Treatment. Environ. Technol. 2011, 32, 971–977. [Google Scholar] [CrossRef]
- Abidin, Z.Z.; Madehi, N.; Yunus, R.; Derahman, A. Effect of Storage Conditions on Jatropha curcas Performance as Biocoagulant for Treating Palm Oil Mill Effluent. J. Environ. Sci. Technol. 2019, 12, 92–101. [Google Scholar] [CrossRef]
- Sibartie, S.; Ismail, N. Potential of Hibiscus Sabdariffa and Jatropha curcas as Natural Coagulants in the Treatment of Pharmaceutical Wastewater. MATEC Web Conf. 2018, 152, 01009. [Google Scholar] [CrossRef]
- Nacke, H.; Gonçalves, A.C.; Coelho, G.F.; Schwantes, D.; Campagnolo, M.A.; Leismann, E.A.V.; Junior, É.C.; Miola, A.J. Removal of Cd (II) from Water Using the Waste of Jatropha Fruit (Jatropha curcas L.). Appl. Water Sci. 2017, 7, 3207–3222. [Google Scholar] [CrossRef]
- Pudza, M.Y.; Abidin, Z.Z. A Sustainable and Eco-Friendly Technique for Dye Adsorption from Aqueous Media Using Waste from Jatropha curcas (Isotherm and Kinetic Model). DWT 2020, 182, 365–374. [Google Scholar] [CrossRef]
- Harun, N.H.; Zainal Abidin, Z.; Abdullah, A.H.; Othaman, R. Sustainable Jatropha Oil-Based Membrane with Graphene Oxide for Potential Application in Cu(II) Ion Removal from Aqueous Solution. Processes 2020, 8, 230. [Google Scholar] [CrossRef]
- Hadi, S.; Mohammed, A.A.; Al-Jubouri, S.M.; Abd, M.F.; Majdi, H.S.; Alsalhy, Q.F.; Rashid, K.T.; Ibrahim, S.S.; Salih, I.K.; Figoli, A. Experimental and Theoretical Analysis of Lead Pb2+ and Cd2+ Retention from a Single Salt Using a Hollow Fiber PES Membrane. Membranes 2020, 10, 136. [Google Scholar] [CrossRef]
- Woldehawariat, B.S. Flux And Rejection Characteristics Of Amine Wastewater Using Membrane Separation Processes. Master’s Thesis, Universiti Teknologi Petronas, Seri Iskandar, Malaysia, 2010. [Google Scholar]
- Bellona, C.; Drewes, J.E.; Xu, P.; Amy, G. Factors Affecting the Rejection of Organic Solutes during NF/RO Treatment—A Literature Review. Water Res. 2004, 38, 2795–2809. [Google Scholar] [CrossRef] [PubMed]
- Nghiem, L.D.; Schäfer, A.I.; Elimelech, M. Role of Electrostatic Interactions in the Retention of Pharmaceutically Active Contaminants by a Loose Nanofiltration Membrane. J. Membr. Sci. 2006, 286, 52–59. [Google Scholar] [CrossRef]
- You, S.; Lu, J.; Tang, C.Y.; Wang, X. Rejection of Heavy Metals in Acidic Wastewater by a Novel Thin-Film Inorganic Forward Osmosis Membrane. Chem. Eng. J. 2017, 320, 532–538. [Google Scholar] [CrossRef]
- Issa, M.A.; Abidin, Z.Z.; Sobri, S.; Rashid, S.A.; Mahdi, M.A.; Ibrahim, N.A. Fluorescent Recognition of Fe3+ in Acidic Environment by Enhanced-Quantum Yield N-Doped Carbon Dots: Optimization of Variables Using Central Composite Design. Sci. Rep. 2020, 10, 11710. [Google Scholar] [CrossRef]
- Stumm, W.; Morgan, J.J. Aquatic Chemistry: Chemical Equilibria and Rates in Natural Waters. In Environmental Science and Technology, 3rd ed.; Wiley & Sons: New York, NY, USA, 1996; ISBN 978-0-471-51184-7. [Google Scholar]
- Hunter, R.J. Introduction to Modern Colloid Science, 4th ed.; University Press: New York, NY, USA, 1993. [Google Scholar]
- Hunter, R.J. Foundations of Colloid Science, 1st ed.; Oxford University Press: Oxford, UK, 2001; ISBN 978-0-19-850502-0. [Google Scholar]
- Harun, N.H. Jatropha Oil-Based Membrane For Cu(II) Ion Removal from Aqueous Solution. Ph.D. Thesis, Universiti Putra Malaysia, Serdang, Selangor, Malaysia, 2022. [Google Scholar]
- Halim, N.A.A.; Abidin, Z.Z.; Siajam, S.I.; Hean, C.G.; Harun, M.R. Optimization Studies and Compositional Analysis of Subcritical Water Extraction of Essential Oil from Citrus Hystrix DC. Leaves. J. Supercrit. Fluids 2021, 178, 105384. [Google Scholar] [CrossRef]
- Ong, S.L.; Zhou, W.; Lianfa, S.; Ng, W.J. Evaluation of Feed Concentration Effects on Salt/Ion Transport through RO/NF Membranes with the Nernst-Planck-Donnan Model. Environ. Eng. Sci. 2002, 19, 11. [Google Scholar] [CrossRef]
- Bowen, W.R.; Mohammad, A.W. Characterization and Prediction of Nanofiltration Membrane Performance—A General Assessment. Trans Ichem 1998, 76, 885. [Google Scholar] [CrossRef]
- Donnan, F.G. Theory of Membrane Equilibria and Membrane Potentials in the Presence of Non-Dialyzing Electrolytes, a Contribution to Physical-Chemical Physiology. J. Membr. Sci. 1995, 100, 45. [Google Scholar] [CrossRef]
- Marecka-Migacz, A.; Mitkowski, P.T.; Nędzarek, A.; Różański, J.; Szaferski, W. Effect of PH on Total Volume Membrane Charge Density in the Nanofiltration of Aqueous Solutions of Nitrate Salts of Heavy Metals. Membranes 2020, 10, 235. [Google Scholar] [CrossRef]
- Tsuru, T.; Urairi, M.; Nakao, S.I.; Kimura, S. Reverse Osmosis of Single and Mixed Electrolytes with Charged Membranes: Experiment and Analysis. J. Chem. Eng. Jpn. 1991, 24, 518. [Google Scholar] [CrossRef]
- Peeters, J.M.M.; Boom, J.P.; Mulder, M.H.V.; Strathmann, H. Retention Measurements of Nanofiltration Membranes with Electrolyte Solutions. J. Membr. Sci. 1998, 145, 199–209. [Google Scholar] [CrossRef]
- Takagi, R.; Larbot, A.; Cot, L.; Nakagaki, M. Effect of Al2O3 Support on Electrical Properties of TiO2/Al2O3 Membrane Formed by Sol–Gel Method. J. Membr. Sci. 2000, 177, 33–40. [Google Scholar] [CrossRef]
- Bowen, W.R.; Mukhtar, H. Characterisation and Prediction of Separation Performance of Nanofiltration Membranes. J. Membr. Sci. 1996, 112, 263–274. [Google Scholar] [CrossRef]
- Abdullah, N.; Rahman, M.A.; Dzarfan Othman, M.H.; Jaafar, J.; Ismail, A.F. Membranes and Membrane Processes. In Current Trends and Future Developments on (Bio-) Membranes; Elsevier: Amsterdam, The Netherlands, 2018; pp. 45–70. ISBN 978-0-12-813549-5. [Google Scholar]
- Qadir, D.; Mukhtar, H.B.; Keong, L.K. Rejection of Divalent Ions in Commercial Tubular Membranes: Effect of Feed Concentration and Anion Type. Sustain. Environ. Res. 2017, 27, 103–106. [Google Scholar] [CrossRef]
- Emamjomeh, M.M.; Torabi, H.; Mousazadeh, M.; Alijani, M.H.; Gohari, F. Impact of Independent and Non-Independent Parameters on Various Elements’ Rejection by Nanofiltration Employed in Groundwater Treatment. Appl. Water Sci. 2019, 9, 71. [Google Scholar] [CrossRef]
- Hiemenz, P.C.; Rajagopalan, R. Principles of Colloid and Surface Chemistry, 3rd ed.; CRC Press: Boca Raton, FL, USA, 1997. [Google Scholar]
- Hunter, R.J. Zeta Potential in Colloid Science: Principles and Applications; Academic Press: Cambridge, MA, USA, 1981. [Google Scholar]
- Cai, H.; Fan, H.; Zhao, L.; Hong, H.; Shen, L.; He, Y.; Lin, H.; Chen, J. Effects of Surface Charge on Interfacial Interactions Related to Membrane Fouling in a Submerged Membrane Bioreactor Based on Thermodynamic Analysis. J. Colloid Interface Sci. 2016, 465, 33–41. [Google Scholar] [CrossRef]
- Kumar, M.; Ulbricht, M. Novel Ultrafiltration Membranes with Adjustable Charge Density Based on Sulfonated Poly(Arylene Ether Sulfone) Block Copolymers and Their Tunable Protein Separation Performance. Polymer 2014, 55, 354–365. [Google Scholar] [CrossRef]
- Xiao, K.; Wang, X.; Huang, X.; Waite, T.D.; Wen, X. Combined Effect of Membrane and Foulant Hydrophobicity and Surface Charge on Adsorptive Fouling during Microfiltration. J. Membr. Sci. 2011, 373, 140–151. [Google Scholar] [CrossRef]
- Lu, G.W.; Gao, P. Emulsions and Microemulsions for Topical and Transdermal Drug Delivery. In Handbook of Non-Invasive Drug Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2010; pp. 59–94. ISBN 978-0-8155-2025-2. [Google Scholar]
- Breite, D.; Went, M.; Prager, A.; Schulze, A. The Critical Zeta Potential of Polymer Membranes: How Electrolytes Impact Membrane Fouling. RSC Adv. 2016, 6, 98180–98189. [Google Scholar] [CrossRef]
- Nguyen, P.T. Role of Charge Effects during Membrane Filtration. Master’s Thesis, Universiteit Gent, Ghent, Belgium, 2013. [Google Scholar]
- Elimelech, M.; Childress, A.E. Zeta Potential of Reverse Osmosis Membranes: Implications for Membrane Perfromance; Water Treatment Technology Program Report; Water Treatment Engineering and Reserch Group, Department of Civil and Environmental Engineering, Universiti of California: Los Angeles, CA, USA, 1996; p. 71. [Google Scholar]
- Stumm, W.; Sigg, L.; Sulzberger, B. Chemistry of the Solid-Water Interface: Processes at the Mineral-Water and Particle-Water Interface in Natural Systems; Wiley & Sons: New York, NY, USA, 1992; ISBN 978-0-471-57672-3. [Google Scholar]
- Grancarić, A.M.; Ristić, N.; Tarbuk, A.; Ristić, I. Electrokinetic Phenomena of Cationized Cotton and Its Dyeability with Reactive Dyes. Fibres Text. East. Eur. 2013, 6, 106–110. [Google Scholar]
- Behera, S.K.; Meena, H.; Chakraborty, S.; Meikap, B.C. Application of Response Surface Methodology (RSM) for Optimization of Leaching Parameters for Ash Reduction from Low-Grade Coal. Int. J. Min. Sci. Technol. 2018, 28, 621–629. [Google Scholar] [CrossRef]
- Javanbakht, V.; Ghoreishi, S.M. Application of Response Surface Methodology for Optimization of Lead Removal from an Aqueous Solution by a Novel Superparamagnetic Nanocomposite. Adsorpt. Sci. Technol. 2017, 35, 241–260. [Google Scholar] [CrossRef]
- Yalodah, D. The Concise Encyclopedia of Statistics; Springer: New York, NY, USA, 2008; ISBN 978-0-387-31742-7. [Google Scholar]
- Minitab Multiple Regression Analysis: Use Adjusted R-Squared and Predicted R-Squared to Include the Correct Number of Variables. Available online: https://blog.minitab.com/en/adventures-in-statistics-2/multiple-regession-analysis-use-adjusted-r-squared-and-predicted-r-squared-to-include-the-correct-number-of-variables (accessed on 11 February 2022).
- Rajewski, J.; Dobrzyńska-Inger, A. Application of Response Surface Methodology (RSM) for the Optimization of Chromium(III) Synergistic Extraction by Supported Liquid Membrane. Membranes 2021, 11, 854. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.J.; Whitcomb, P.J. RSM Simplified: Optimizing Processes Using Response Surface Methods for Design of Experiments, 2nd ed.; Taylor & Francis: Boca Raton, FL, USA, 2017; ISBN 978-1-4987-4598-7. [Google Scholar]
- Vladić, J.; Canli, O.; Pavlić, B.; Zeković, Z.; Vidović, S.; Kaplan, M. Optimization of Satureja Montana Subcritical Water Extraction Process and Chemical Characterization of Volatile Fraction of Extracts. J. Supercrit. Fluids 2017, 120, 86–94. [Google Scholar] [CrossRef]
- Zeković, Z.; Kaplan, M.; Pavlić, B.; Olgun, E.O.; Vladić, J.; Canlı, O.; Vidović, S. Chemical Characterization of Polyphenols and Volatile Fraction of Coriander (Coriandrum sativum L.) Extracts Obtained by Subcritical Water Extraction. Ind. Crops Prod. 2016, 87, 54–63. [Google Scholar] [CrossRef]
- Carley, K.M.; Kamneva, N.Y.; Reminga, J. Response Surface Methodology; Carnegie Mellon University: Pittsburgh, PA, USA, 2004; p. 13. [Google Scholar]
- Tiwari, A.; Pal, D.; Sahu, O. Recovery of Copper from Synthetic Solution by Efficient Technology: Membrane Separation with Response Surface Methodology. Resour. Effic. Technol. 2017, 3, 37–45. [Google Scholar] [CrossRef]
- Vera Candioti, L.; De Zan, M.M.; Cámara, M.S.; Goicoechea, H.C. Experimental Design and Multiple Response Optimization. Using the Desirability Function in Analytical Methods Development. Talanta 2014, 124, 123–138. [Google Scholar] [CrossRef] [PubMed]
- Vining, G. Technical Advice: Residual Plots to Check Assumptions. Qual. Eng. 2010, 23, 105–110. [Google Scholar] [CrossRef]











| Symbol | Factor | Low Level | Middle | High Level |
|---|---|---|---|---|
| A | Concentration (ppm) | 60 | 100 | 140 |
| B | Pressure (bar) | 1.5 | 2 | 2.5 |
| C | pH value | 3 | 4 | 5 |
| No. | Factor A: Concentration (ppm) | Factor B: Pressure (bar) | Factor C: pH | Response 1: Cu(II) Ions Rejection (%) |
|---|---|---|---|---|
| 1 | 60 | 2.0 | 4 | 70 |
| 2 | 60 | 2.5 | 5 | 58 |
| 3 | 100 | 1.5 | 4 | 82 |
| 4 | 100 | 2.0 | 3 | 80 |
| 5 | 100 | 2.0 | 4 | 87 |
| 6 | 100 | 2.0 | 4 | 81 |
| 7 | 100 | 2.0 | 4 | 81 |
| 8 | 100 | 2.0 | 5 | 58 |
| 9 | 100 | 2.5 | 4 | 68 |
| 10 | 140 | 1.5 | 3 | 78 |
| 11 | 140 | 1.5 | 5 | 66 |
| 12 | 140 | 2.0 | 4 | 60 |
| 13 | 140 | 2.5 | 3 | 30 |
| 14 | 140 | 2.5 | 5 | 15 |
| Source | Sum of Squares | Df | Mean Square | F-Value | p-Value Prob > F | Remarks |
|---|---|---|---|---|---|---|
| Mean | 59,800.48 | 1 | 59,800.48 | - | - | - |
| Linear | 4054.09 | 3 | 1351.36 | 8.80 | 0.0037 | Suggested |
| 2FI | 370.89 | 3 | 123.63 | 0.74 | 0.5596 | - |
| Quadratic | 1096.34 | 3 | 365.45 | 21.32 | 0.0064 | Suggested |
| Cubic | 42.94 | 2 | 21.47 | 1.68 | 0.3737 | Aliased |
| Residual | 25.63 | 2 | 12.81 | - | - | - |
| Total | 65,390.37 | 14 | 4670.74 | - | - | - |
| Source | Sum of Squares | Df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 55.27 | 9 | 613.48 | 35.79 | 0.0018 |
| A (Concentration) | 79.56 | 1 | 55.27 | 3.22 | 0.1470 |
| B (Pressure) | 275.13 | 1 | 79.56 | 4.64 | 0.0975 |
| C (Ph) | 722.57 | 1 | 275.13 | 16.05 | 0.0160 |
| AB | 14.14 | 1 | 722.57 | 42.15 | 0.0029 |
| AC | 1.16 | 1 | 14.14 | 0.82 | 0.4152 |
| BC | 486.37 | 1 | 1.16 | 0.068 | 0.8075 |
| A2 | 53.68 | 1 | 486.37 | 28.37 | 0.0060 |
| B2 | 253.67 | 1 | 53.68 | 3.13 | 0.1515 |
| C2 | 55.27 | 1 | 253.67 | 14.80 | 0.0184 |
| Residual | 68.57 | 4 | 17.14 | ||
| Lack of fit (LOF) | 42.94 | 2 | 21.47 | 1.68 | 0.3737 |
| Pure error | 25.63 | 2 | 12.81 | ||
| Corrected total | 5589.89 | 13 | |||
| Statistical analysis of the regression equation | |||||
| R2 | 0.9877 | Standard deviation (SD) | 4.14 | ||
| Adjusted-R2 | 0.9601 | Mean | 65.36 | ||
| Predicted-R2 | −0.1643 | Coefficient of variation (CV%) | 6.34 | ||
| Adequate precision | 19.358 | PRESS | 6508.51 | ||
| Factor/ Response | Level | Optimization Goal | Lower Limit | Upper Limit | Prediction Point |
|---|---|---|---|---|---|
| A | Concentration (ppm) | In range | 60 | 140 | 116 |
| B | Pressure (bar) | In range | 1.5 | 2.5 | 1.5 |
| C | Acidity (pH) | In range | 3 | 5 | 3.7 |
| Response Y | Cu(II) ions Rejection (%) | Maximize | 14 | 87 | 87 |
| Predicted Response | Desirability | 95% PI Low | 95% PI High | Observed Response | Error (%) |
|---|---|---|---|---|---|
| 87 | 1 | 76 | 99 | 82 | 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harun, N.H.; Zainal Abidin, Z.; Majid, U.A.; Abdul Hamid, M.R.; Abdullah, A.H.; Othaman, R.; Harun, M.Y. Adopting Sustainable Jatropha Oil Bio-Based Polymer Membranes as Alternatives for Environmental Remediation. Polymers 2022, 14, 3325. https://doi.org/10.3390/polym14163325
Harun NH, Zainal Abidin Z, Majid UA, Abdul Hamid MR, Abdullah AH, Othaman R, Harun MY. Adopting Sustainable Jatropha Oil Bio-Based Polymer Membranes as Alternatives for Environmental Remediation. Polymers. 2022; 14(16):3325. https://doi.org/10.3390/polym14163325
Chicago/Turabian StyleHarun, Nur Haninah, Zurina Zainal Abidin, Umar Adam Majid, Mohamad Rezi Abdul Hamid, Abdul Halim Abdullah, Rizafizah Othaman, and Mohd Yusof Harun. 2022. "Adopting Sustainable Jatropha Oil Bio-Based Polymer Membranes as Alternatives for Environmental Remediation" Polymers 14, no. 16: 3325. https://doi.org/10.3390/polym14163325
APA StyleHarun, N. H., Zainal Abidin, Z., Majid, U. A., Abdul Hamid, M. R., Abdullah, A. H., Othaman, R., & Harun, M. Y. (2022). Adopting Sustainable Jatropha Oil Bio-Based Polymer Membranes as Alternatives for Environmental Remediation. Polymers, 14(16), 3325. https://doi.org/10.3390/polym14163325