Synthesis and Evaluation of Thermoresponsive Renewable Lipid-Based Block Copolymers for Drug Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of 2-(Methacryloyloxy) Ethyl Stearate (SAMA)
2.3. Synthesis of Homopolymers
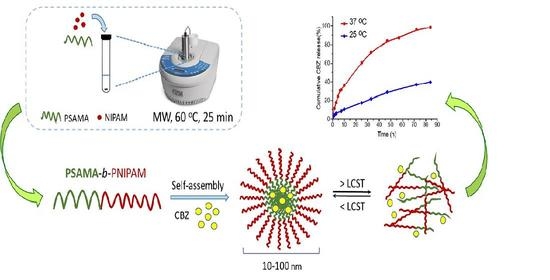
2.4. Synthesis of PSAMA-b-PNIPAM Block Copolymer
2.5. Characterization and Instruments
2.6. Determination of Critical Micelle Concentration (CMC)
2.7. Micelle Formation
2.8. Drug Loading within Micelles
2.9. In Vitro Drug Release Study
3. Results and Discussion
3.1. Synthesis of Block Copolymer PSAMA-b-PNIPAM
3.2. Characterization of PSAMA-b-PNIPAM
3.3. Self-Assembly Study of PSAMA-b-PNIPAM
3.4. Critical Micelle Concentration (CMC) Determination
3.5. Drug Loading and Release Behavior of Block Copolymer PSAMA-b-PNIPAM
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alavi, M.; Karimi, N.; Safaei, M. Application of Various Types of Liposomes in Drug Delivery Systems. Adv. Pharm. Bull. 2017, 7, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Kesharwani, P.; Jain, K.; Jain, N.K. Dendrimer as Nanocarrier for Drug Delivery. Prog. Polym. Sci. 2014, 39, 268–307. [Google Scholar] [CrossRef]
- Huang, H.; Yuan, Q.; Shah, J.S.; Misra, R.D.K. A New Family of Folate-Decorated and Carbon Nanotube-Mediated Drug Delivery System: Synthesis and Drug Delivery Response. Adv. Drug Deliv. Rev. 2011, 63, 1332–1339. [Google Scholar] [CrossRef] [PubMed]
- Din, F.U.; Aman, W.; Ullah, I.; Qureshi, O.S.; Mustapha, O.; Shafique, S.; Zeb, A. Effective Use of Nanocarriers as Drug Delivery Systems for the Treatment of Selected Tumors. Int. J. Nanomed. 2017, 12, 7291–7309. [Google Scholar] [CrossRef] [Green Version]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007, 2, 751–760. [Google Scholar] [CrossRef]
- Ghosh, B.; Biswas, S. Polymeric Micelles in Cancer Therapy: State of the Art. J. Control. Release 2021, 332, 127–147. [Google Scholar] [CrossRef]
- Kulthe, S.S.; Choudhari, Y.M.; Inamdar, N.N.; Mourya, V. Polymeric Micelles: Authoritative Aspects for Drug Delivery. Des. Monomers Polym. 2012, 15, 465–521. [Google Scholar] [CrossRef]
- Kedar, U.; Phutane, P.; Shidhaye, S.; Kadam, V. Advances in Polymeric Micelles for Drug Delivery and Tumor Targeting. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 714–729. [Google Scholar] [CrossRef]
- Torchilin, V. Tumor Delivery of Macromolecular Drugs Based on the EPR Effect. Adv. Drug Deliv. Rev. 2011, 63, 131–135. [Google Scholar] [CrossRef]
- Hwang, D.; Ramsey, J.D.; Kabanov, A.v. Polymeric Micelles for the Delivery of Poorly Soluble Drugs: From Nanoformulation to Clinical Approval. Adv. Drug Deliv. Rev. 2020, 156, 80–118. [Google Scholar] [CrossRef]
- Lu, Y.; Park, K. Polymeric Micelles and Alternative Nanonized Delivery Vehicles for Poorly Soluble Drugs. Int. J. Pharm. 2013, 453, 198–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Chan, H.F.; Leong, K.W. Advanced Materials and Processing for Drug Delivery: The Past and the Future. Adv. Drug Deliv. Rev. 2013, 65, 104–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.; Kim, J.H.; Jeon, O.; Kwon, I.C.; Park, K. Engineered Polymers for Advanced Drug Delivery. Eur. J. Pharm. Biopharm. 2009, 71, 420–430. [Google Scholar] [CrossRef] [Green Version]
- Shahriari, M.; Zahiri, M.; Abnous, K.; Taghdisi, S.M.; Ramezani, M.; Alibolandi, M. Enzyme Responsive Drug Delivery Systems in Cancer Treatment. J. Control. Release 2019, 308, 172–189. [Google Scholar] [CrossRef] [PubMed]
- Popat, A.; Liu, J.; Lu, G.Q.; Qiao, S.Z. A PH-Responsive Drug Delivery System Based on Chitosan Coated Mesoporous Silica Nanoparticles. J. Mater. Chem. 2012, 22, 11173–11178. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, R.; Yang, M.; Jiang, X.; Liu, B. Thermo and PH Dual-Responsive Nanoparticles for Anti-Cancer Drug Delivery. Adv. Mater. 2007, 19, 2988–2992. [Google Scholar] [CrossRef]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-Responsive Nanocarriers for Drug Delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef]
- Raza, A.; Rasheed, T.; Nabeel, F.; Hayat, U.; Bilal, M.; Iqbal, H.M.N. Endogenous and Exogenous Stimuli-Responsive Drug Delivery Systems for Programmed Site-Specific Release. Molecules 2019, 24, 1117. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Du, H.; Zhang, W.; Zhai, G. Internal Stimuli-Responsive Nanocarriers for Drug Delivery: Design Strategies and Applications. Mater. Sci. Eng. C 2017, 71, 1267–1280. [Google Scholar] [CrossRef]
- Lue, S.J.; Chen, C.-H.; Shih, C.-M. Tuning of Lower Critical Solution Temperature (LCST) of Poly(N-Isopropylacrylamide-Co-Acrylic Acid) Hydrogels. J. Macromol. Sci. Part B Phys. 2011, 50, 563–579. [Google Scholar] [CrossRef]
- Dalgakiran, E.; Tatlipinar, H. The Role of Hydrophobic Hydration in the LCST Behaviour of POEGMA300 by All-Atom Molecular Dynamics Simulations. Phys. Chem. Chem. Phys. 2018, 20, 15389–15399. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.Q.; Wang, T.X.; Liu, W.; Wang, C.D.; Wang, D.; Shang, T.; Shen, L.H.; Ren, L. Multifunctional Au@IPN-PNIPAAm Nanogels for Cancer Cell Imaging and Combined Chemo-Photothermal Treatment. J. Mater. Chem. 2011, 21, 7240–7247. [Google Scholar] [CrossRef]
- Moran, M.T.; Carroll, W.M.; Selezneva, I.; Gorelov, A.; Rochev, Y. Cell Growth and Detachment from Protein-Coated PNIPAAm-Based Copolymers. J. Biomed. Mater. Res. Part A 2007, 81, 870–876. [Google Scholar] [CrossRef] [PubMed]
- Lanzalaco, S.; Armelin, E. Poly(N-Isopropylacrylamide) and Copolymers: A Review on Recent Progresses in Biomedical Applications. Gels 2017, 3, 36. [Google Scholar] [CrossRef]
- Qian, S.; Liu, R.; Han, G.; Shi, K.; Zhang, W. Star Amphiphilic Block Copolymers: Synthesis: Via Polymerization-Induced Self-Assembly and Crosslinking within Nanoparticles, and Solution and Interfacial Properties. Polym. Chem. 2020, 11, 2532–2541. [Google Scholar] [CrossRef]
- Li, Y.Y.; Zhang, X.Z.; Zhu, J.L.; Cheng, H.; Cheng, S.X.; Zhuo, R.X. Self-Assembled, Thermoresponsive Micelles Based on Triblock PMMA-b-PNIPAAm-b-PMMA Copolymer for Drug Delivery. Nanotechnology 2007, 18, 215605. [Google Scholar] [CrossRef]
- Chang, C.; Wei, H.; Quan, C.Y.; Li, Y.Y.; Liu, J.; Wang, Z.C.; Cheng, S.X.; Zhang, X.Z.; Zhuo, R.X. Fabrication of Thermosensitive PCL-PNIPAAm-PCL Triblock Copolymeric Micelles for Drug Delivery. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 3048–3057. [Google Scholar] [CrossRef]
- Lin, W.; Ma, G.; Kampf, N.; Yuan, Z.; Chen, S. Development of Long-Circulating Zwitterionic Cross-Linked Micelles for Active-Targeted Drug Delivery. Biomacromolecules 2016, 17, 2010–2018. [Google Scholar] [CrossRef]
- Sandker, M.J.; Duque, L.F.; Redout, E.M.; Chan, A.; Que, I.; Löwik, C.W.G.M.; Klijnstra, E.C.; Kops, N.; Steendam, R.; van Weeren, R.; et al. Degradation, Intra-Articular Retention and Biocompatibility of Monospheres Composed of [PDLLA-PEG-PDLLA]-b-PLLA Multi-Block Copolymers. Acta Biomater. 2017, 48, 401–414. [Google Scholar] [CrossRef]
- Wang, S.; Vajjala Kesava, S.; Gomez, E.D.; Robertson, M.L. Sustainable Thermoplastic Elastomers Derived from Fatty Acids. Macromolecules 2013, 46, 7202–7212. [Google Scholar] [CrossRef]
- Maisonneuve, L.; Lebarbé, T.; Grau, E.; Cramail, H. Structure-Properties Relationship of Fatty Acid-Based Thermoplastics as Synthetic Polymer Mimics. Polym. Chem. 2013, 4, 5472–5517. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Zhang, C.; Madbouly, S.A. In Situ Polymerization of Bio-Based Thermosetting Polyurethane/Graphene Oxide Nanocomposites. J. Appl. Polym. Sci. 2015, 132, 41751. [Google Scholar] [CrossRef]
- György, C.; Hunter, S.J.; Girou, C.; Derry, M.J.; Armes, S.P. Synthesis of Poly(Stearyl Methacrylate)-Poly(2-Hydroxypropyl Methacrylate) Diblock Copolymer Nanoparticles: Via RAFT Dispersion Polymerization of 2-Hydroxypropyl Methacrylate in Mineral Oil. Polym. Chem. 2020, 11, 4579–4590. [Google Scholar] [CrossRef]
- Obeng, M.; Milani, A.H.; Musa, M.S.; Cui, Z.; Fielding, L.A.; Farrand, L.; Goulding, M.; Saunders, B.R. Self-Assembly of Poly(Lauryl Methacrylate)-b-Poly(Benzyl Methacrylate) Nano-Objects Synthesised by ATRP and Their Temperature-Responsive Dispersion Properties. Soft Matter 2017, 13, 2228–2238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maiti, B.; De, P. RAFT Polymerization of Fatty Acid Containing Monomers: Controlled Synthesis of Polymers from Renewable Resources. RSC Adv. 2013, 3, 24983–24990. [Google Scholar] [CrossRef] [Green Version]
- Jena, S.S.; Roy, S.G.; Azmeera, V.; De, P. Solvent-Dependent Self-Assembly Behaviour of Block Copolymers Having Side-Chain Amino Acid and Fatty Acid Block Segments. React. Funct. Polym. 2016, 99, 26–34. [Google Scholar] [CrossRef]
- Zhao, X.; Shan, G. PSMA-b-PNIPAM Copolymer Micelles with Both a Hydrophobic Segment and a Hydrophilic Terminal Group: Synthesis, Micelle Formation, and Characterization. Colloid Polym. Sci. 2019, 297, 1353–1363. [Google Scholar] [CrossRef]
- Arshad, M.; Pradhan, R.A.; Ullah, A. Synthesis of Lipid-Based Amphiphilic Block Copolymer and Its Evaluation as Nano Drug Carrier. Mater. Sci. Eng. C 2017, 76, 217–223. [Google Scholar] [CrossRef]
- Chang, L.; Liu, J.; Zhang, J.; Deng, L.; Dong, A. PH-Sensitive Nanoparticles Prepared from Amphiphilic and Biodegradable Methoxy Poly(Ethylene Glycol)-Block-(Polycaprolactone-Graft-Poly(Methacrylic Acid)) for Oral Drug Delivery. Polym. Chem. 2013, 4, 1430–1438. [Google Scholar] [CrossRef]
- Zhang, S.; Arshad, M.; Ullah, A. Drug Encapsulation and Release Behavior of Telechelic Nanoparticles. Nanotechnology 2015, 26, 415703. [Google Scholar] [CrossRef]
- Huh, K.M.; Lee, S.C.; Cho, Y.W.; Lee, J.; Jeong, J.H.; Park, K. Hydrotropic Polymer Micelle System for Delivery of Paclitaxel. J. Control. Release 2005, 101, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Wu, J.; Zhou, H.; Qu, Y.; Li, B.; Zhang, W. Self-Assembled Blends of AB/BAB Block Copolymers Prepared through Dispersion RAFT Polymerization. Macromolecules 2016, 49, 4490–4500. [Google Scholar] [CrossRef]
- York, A.W.; Kirkland, S.E.; McCormick, C.L. Advances in the Synthesis of Amphiphilic Block Copolymers via RAFT Polymerization: Stimuli-Responsive Drug and Gene Delivery. Adv. Drug Deliv. Rev. 2008, 60, 1018–1036. [Google Scholar] [CrossRef] [PubMed]
- Wan, W.M.; Hong, C.Y.; Pan, C.Y. One-Pot Synthesis of Nanomaterials via RAFT Polymerization Induced Self-Assembly and Morphology Transition. Chem. Commun. 2009, 39, 5883–5885. [Google Scholar] [CrossRef]
- Craig, A.F.; Clark, E.E.; Sahu, I.D.; Zhang, R.; Frantz, N.D.; Al-Abdul-Wahid, M.S.; Dabney-Smith, C.; Konkolewicz, D.; Lorigan, G.A. Tuning the Size of Styrene-Maleic Acid Copolymer-Lipid Nanoparticles (SMALPs) Using RAFT Polymerization for Biophysical Studies. Biochim. Biophys. Acta Biomembr. 2016, 1858, 2931–2939. [Google Scholar] [CrossRef]
- Perrier, S. 50th Anniversary Perspective: RAFT Polymerization-A User Guide. Macromolecules 2017, 50, 7433–7447. [Google Scholar] [CrossRef]
- Uemukai, T.; Hioki, T.; Ishifune, M. Thermoresponsive and Redox Behaviors of Poly(N -Isopropylacrylamide)-Based Block Copolymers Having TEMPO Groups as Their Side Chains. Int. J. Polym. Sci. 2013, 2013, 196145. [Google Scholar] [CrossRef] [Green Version]
- Vega-Rios, A.; Licea-Claveríe, A. Controlled Synthesis of Block Copolymers Containing N-Isopropylacrylamide by RAFT Polymerization. J. Mex. Chem. Soc. 2011, 55, 21–32. [Google Scholar] [CrossRef]
- Sumerlin, B.S.; Lowe, A.B.; Thomas, D.B.; Convertine, A.J.; Donovan, M.S.; Mccormick, C.L. Aqueous Solution Properties of PH-Responsive AB Diblock Acrylamido-Styrenic Copolymers Synthesized via Aqueous Reversible Addition-Fragmentation Chain Transfer. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 1724–1734. [Google Scholar] [CrossRef]
- Rizzardo, E.; Chen, M.; Chong, B.; Moad, G.; Skidmore, M.; Thang, S.H. RAFT Polymerization: Adding to the Picture. Macromol. Symp. 2007, 248, 104–116. [Google Scholar] [CrossRef]
- Maiti, B.; Maiti, S.; De, P. Self-Assembly of Well-Defined Fatty Acid Based Amphiphilic Thermoresponsive Random Copolymers. RSC Adv. 2016, 6, 19322–19330. [Google Scholar] [CrossRef]
- Zhai, S.; Ma, Y.; Chen, Y.; Li, D.; Cao, J.; Liu, Y.; Cai, M.; Xie, X.; Chen, Y.; Luo, X. Synthesis of an Amphiphilic Block Copolymer Containing Zwitterionic Sulfobetaine as a Novel PH-Sensitive Drug Carrier. Polym. Chem. 2014, 5, 1285–1297. [Google Scholar] [CrossRef]
- Kakde, D.; Taresco, V.; Bansal, K.K.; Magennis, E.P.; Howdle, S.M.; Mantovani, G.; Irvine, D.J.; Alexander, C. Amphiphilic Block Copolymers from a Renewable ϵ-Decalactone Monomer: Prediction and Characterization of Micellar Core Effects on Drug Encapsulation and Release. J. Mater. Chem. B 2016, 4, 7119–7129. [Google Scholar] [CrossRef] [PubMed]
- Schmelz, J.; Schedl, A.E.; Steinlein, C.; Manners, I.; Schmalz, H. Length Control and Block-Type Architectures in Worm-like Micelles with Polyethylene Cores. J. Am. Chem. Soc. 2012, 134, 14217–14225. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Gohy, J.F.; Willet, N.; Zhang, J.X.; Varshney, S.; Jérôme, R. Tuning of the Morphology of Core-Shell-Corona Micelles in Water. I. Transition from Sphere to Cylinder. Macromolecules 2004, 37, 1089–1094. [Google Scholar] [CrossRef]
- Ott, C.; Hoogenboom, R.; Hoeppener, S.; Wouters, D.; Gohy, J.F.; Schubert, U.S. Tuning the Morphologies of Amphiphilic Metallo-Supramolecular Triblock Terpolymers: From Spherical Micelles to Switchable Vesicles. Soft Matter 2009, 5, 84–91. [Google Scholar] [CrossRef]
- Yokoyama, M. Polymeric Micelles as Drug Carriers: Their Lights and Shadows. J. Drug Target. 2014, 22, 576–583. [Google Scholar] [CrossRef]
- Ghezzi, M.; Pescina, S.; Padula, C.; Santi, P.; del Favero, E.; Cantù, L.; Nicoli, S. Polymeric Micelles in Drug Delivery: An Insight of the Techniques for Their Characterization and Assessment in Biorelevant Conditions. J. Control. Release 2021, 332, 312–336. [Google Scholar] [CrossRef]
- Miyata, K.; Christie, R.J.; Kataoka, K. Polymeric Micelles for Nano-Scale Drug Delivery. React. Funct. Polym. 2011, 71, 227–234. [Google Scholar] [CrossRef]
- Owen, S.C.; Chan, D.P.Y.; Shoichet, M.S. Polymeric Micelle Stability. Nano Today 2012, 7, 53–65. [Google Scholar] [CrossRef]
- Wu, Q.; Yi, J.; Yin, Z.; Wang, S.; Yang, Q.; Wu, S.; Song, X.; Zhang, G. Synthesis and Self-Assembly of New Amphiphilic Thermosensitive Poly(N-Vinylcaprolactam)/Poly(ε-Caprolactone) Block Copolymers via the Combination of Ring-Opening Polymerization and Click Chemistry. J. Polym. Res. 2013, 20, 262. [Google Scholar] [CrossRef]
- Aliabadi, H.M.; Lavasanifar, A. Polymeric Micelles for Drug Delivery. Expert Opin. Drug Deliv. 2006, 3, 139–162. [Google Scholar] [CrossRef]









| Polymer a | [M]/[CTA]/[I] | Time (min) | Conv. b | Mn c (g/mol) | Mw/Mn c | Mn,nmr b (g/mol) | Composition b SAMA: NIPAM |
|---|---|---|---|---|---|---|---|
| PSAMA8 | 25:1:0.2 | 20 | 28.6% | 4730 | 1.08 | 3580 | 100:0 |
| PSAMA12 | 50:1:0.2 | 20 | 23.5% | 6820 | 1.10 | 5160 | 100:0 |
| PSAMA21 * | 25:1:0.2 | 300 | 69.3% | 10,500 | 1.09 | 8610 | 100:0 |
| PNIPAM58 | 150:1:0.2 | 30 | 35.6% | 5640 | 1.06 | 6960 | 0:100 |
| PSAMA8- b-PNIPAM49 | 100:1:0.2 | 25 | 75.6% | 9320 | 1.10 | 9120 | 14:86 |
| PSAMA8- b-PNIPAM106 | 300:1:0.2 | 25 | 62.3% | 11,430 | 1.15 | 15,570 | 7:93 |
| PSAMA12- b-PNIPAM121 | 300:1:0.2 | 25 | 64.1% | 14,570 | 1.18 | 18,860 | 9:91 |
| DLE (%) | DLC (%) | |
|---|---|---|
| PSAMA8-b-PNIPAM49 | 31.6 | 2.9 |
| PSAMA8-b-PNIPAM106 | 24.4 | 2.2 |
| PSAMA12-b-PNIPAM121 | 22.2 | 2.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Ullah, A. Synthesis and Evaluation of Thermoresponsive Renewable Lipid-Based Block Copolymers for Drug Delivery. Polymers 2022, 14, 3436. https://doi.org/10.3390/polym14173436
Wang H, Ullah A. Synthesis and Evaluation of Thermoresponsive Renewable Lipid-Based Block Copolymers for Drug Delivery. Polymers. 2022; 14(17):3436. https://doi.org/10.3390/polym14173436
Chicago/Turabian StyleWang, Huiqi, and Aman Ullah. 2022. "Synthesis and Evaluation of Thermoresponsive Renewable Lipid-Based Block Copolymers for Drug Delivery" Polymers 14, no. 17: 3436. https://doi.org/10.3390/polym14173436
APA StyleWang, H., & Ullah, A. (2022). Synthesis and Evaluation of Thermoresponsive Renewable Lipid-Based Block Copolymers for Drug Delivery. Polymers, 14(17), 3436. https://doi.org/10.3390/polym14173436