Attapulgite Structure Reset to Accelerate the Crystal Transformation of Isotactic Polybutene
Abstract
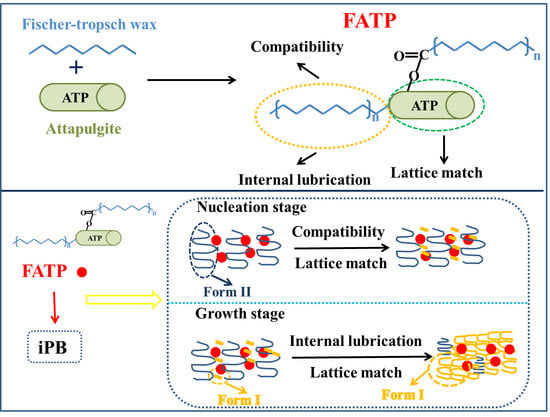
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of FATP
2.3. Characterization of the FATP
2.4. Preparation of iPB/ATP Composite
2.5. Characterization of the iPB/ATP Composite
2.5.1. The Flexural Strength of the iPB/ATP Composite
2.5.2. The Scanning Electron Microscope (SEM) of the iPB/ATP Composite
2.5.3. The Crystal Transformation Behavior of the iPB/ATP Composite
2.5.4. The X-ray Diffraction (XRD) of iPB/ATP Composite
2.5.5. The Polarizing Microscope (POM) of the iPB/ATP Composite
3. Result and Discussion
3.1. The FT-IR Spectra of the Samples
3.2. The X-ray Diffraction of the Samples
3.3. The Hydrophilic Test of the ATP and the FATP
3.4. The Flexural Strength of the iPB/ATP Composite
3.5. The SEM of the iPB/ATP Composite
3.6. The Crystal Transformation Behavior of the iPB/ATP Composite
3.6.1. The Crystal Transformation of the iPB/ATP Composite
3.6.2. The Crystal Transformation of the iPB/ATP Composite in Nucleation Stage
3.6.3. The Crystal Transformation of the iPB/ATP Composite in Growth Stage
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zhong, Z.X.; Su, Z.H. Mechanism of form I' formation in polybutene-1/polypropylene blends. Polymer 2021, 239, 124469. [Google Scholar] [CrossRef]
- Tashiro, K.; Hu, J.; Wang, H.; Hanesaka, M.; Saiani, A. Refinement of the Crystal Structures of Forms I and II of Isotactic Polybutene-1 and a Proposal of Phase Transition Mechanism between Them. Macromolecules 2016, 49, 1392–1404. [Google Scholar] [CrossRef]
- Ma, Y.P.; He, A.H.; Liu, C.J. Crystallization kinetics, crystalline structures and properties of PB/PP blends regulated by poly(butene-block-propylene) copolymers. Polymer 2021, 228, 123901. [Google Scholar] [CrossRef]
- Wang, B.; Lin, F.H.; Zhao, Y.Y.; Li, X.Y.; Liu, Y.C.; Li, J.B.; Han, X.J.; Liu, S.X.; Ji, X.R.; Luo, J.; et al. Isotactic Polybutene-1/Bamboo Powder Composites with Excellent Properties at Initial Stage of Molding. Polymers 2019, 11, 1981. [Google Scholar] [CrossRef] [PubMed]
- Danusso, F.; Gianotti, G. Isotactic polybutene-1: Formation and transformation of modification 2. Macromol. Chem. Phys. 2010, 88, 149–158. [Google Scholar] [CrossRef]
- Wang, Y.T.; Lu, Y.; Zhao, J.Y.; Jiang, Z.Y.; Men, Y.F. Direct Formation of Different Crystalline Forms in Butene-1/Ethylene Copolymer via Manipulating Melt Temperature. Macromolecules 2014, 47, 8653–8662. [Google Scholar] [CrossRef]
- Liu, Y.P.; Cui, K.P.; Tian, N.; Zhou, W.Q.; Meng, L.P.; Li, L.B.; Ma, Z.; Wang, X.L. Stretch-Induced Crystal Crystal Transition of Polybutene-1: An in situ Synchrotron Radiation Wide-Angle X-ray Scattering Study. Macromolecules 2012, 45, 2764–2772. [Google Scholar] [CrossRef]
- Liu, Y.P.; Wang, Y.C.; Zhang, H.Y.; Wu, Y.W.; Zhao, S.; Li, Q.; Shao, C.G.; Wang, Z. Toughening of polybutene-1 with form I' induced by rapid pressurization. Cryst. Eng. Comm. 2021, 24, 300–310. [Google Scholar] [CrossRef]
- Li, L.; Liu, T.; Zhao, L.; Yuan, W.K. CO2 Induced Crystal Phase Transition from Form II to I in Isotactic Poly1-butene. Macromolecules 2009, 42, 2286–2290. [Google Scholar] [CrossRef]
- Qin, Y.N.; Litvinov, V.; Chassé, W.; Zhang, B.; Men, Y.F. Change of lamellar morphology upon polymorphic transition of form II to form I crystals in isotactic Polybutene-1 and its copolymer. Polymer 2020, 215, 123355. [Google Scholar] [CrossRef]
- Wang, B.; Mao, S.D.; Lin, F.H.; Zhang, M.; Zhao, Y.Y.; Zheng, X.H.; Wang, H.; Luo, J. Interfacial Compatibility on the Crystal Transformation of Isotactic Poly (1-Butene)/Herb Residue Composite. Polymers 2021, 13, 1654. [Google Scholar] [CrossRef] [PubMed]
- Idiyatullina, G.K.; Vol’fson, S.I.; Sabirov, R.K.; Yarullin, R.S. Effect of the montmorillonite cloisite 15A on the structure and properties of poly(1-butene). Polym. Sci. Ser. A 2012, 54, 493–498. [Google Scholar] [CrossRef]
- Niu, R.; Gong, J.; Xu, D.H.; Tang, T.; Sun, Z.Y. The effect of particle shape on the structure and rheological properties of carbon-based particle suspensions. Chin. J. Polym. Sci. 2015, 33, 1550–1561. [Google Scholar] [CrossRef]
- Wanjale, S.D.; Jog, J.P. Crystallization and phase transformation kinetics of poly(1-butene)/MWCNT nanocomposites. Polymer 2006, 47, 6414–6421. [Google Scholar] [CrossRef]
- Li, C.; Isshiki, N.; Saito, H.; Kohno, K.; Toyota, A. Nucleation effect of cyclodextrin inclusion complexes on the crystallization of isotactic poly(1-butene). J. Polym. Sci. Part B Polym. Phys. 2010, 48, 389–395. [Google Scholar] [CrossRef]
- Causin, V.; Marega, C.; Marigo, A.; Ferrara, G.; Idiyatullina, G.; Fantinel, F. Morphology, structure and properties of a poly(1-butene)/montmorillonite nanocomposite. Polymer 2006, 47, 4773–4780. [Google Scholar] [CrossRef]
- Qiao, Y.N.; Wang, Q.; Men, Y.F. Kinetics of Nucleation and Growth of Form II to I Polymorphic Transition in Polybutene-1 as Revealed by Stepwise Annealing. Macromolecules 2016, 49, 5126–5136. [Google Scholar] [CrossRef]
- Wittmann, J.C.; Lotz, B. Epitaxial crystallization of polymers on organic and polymeric substrates. Polym. Sci. 1990, 15, 909–948. [Google Scholar] [CrossRef]
- Ouchiar, S.; Stoclet, G.; Cabaret, C.; Gloaguen, V. Influence of the Filler Nature on the Crystalline Structure of Polylactide-Based Nanocomposites: New Insights into the Nucleating Effect. Macromolecules 2016, 49, 2782–2790. [Google Scholar] [CrossRef]
- Su, F.M.; Li, X.Y.; Zhou, W.M.; Chen, W.; Li, H.L.; Cong, Y.H.; Hong, Z.H.; Qi, Z.M.; Li, L.B. Accelerating crystal-crystal transition in poly(1-butene) with two-step crystallization: An in-situ microscopic infrared imaging and microbeam X-ray diffraction study. Polymer 2013, 54, 3408–3416. [Google Scholar] [CrossRef]
- Boor, J.; Mitchell, J.C. Kinetics of crystallization and a crystal-crystal transition in poly-1-butene. J. Polym. Sci. Part A Polym. Chem. 1963, 1, 59–84. [Google Scholar] [CrossRef]
- Di Lorenzo, M.L.; Androsch, R.; Righetti, M.C. The irreversible Form II to Form I transformation in random butene-1/ethylene copolymers. Eur. Polym. J. 2015, 67, 264–273. [Google Scholar] [CrossRef]
- Lu, Z.H.; Hao, Z.Q.; Wang, J.; Chen, L. Efficient removal of europium from aqueous solutions using attapulgite-iron oxide magnetic composites. J. Ind. Eng. Chem. 2016, 34, 374–381. [Google Scholar] [CrossRef]
- Xu, W.B.; He, P.S. Crystallization characteristics of polyoxymethylene with attapulgite as nucleating agent. Polym. Eng. Sci. 2001, 41, 1903–1912. [Google Scholar] [CrossRef]
- Wang, L.H.; Sheng, J.W.; Wu, S. Isothermal Crystallization Kinetics of Polypropylene/Attapulgite Nanocomposites. J. Macromol. Sci. Part B 2004, 43, 935–946. [Google Scholar] [CrossRef]
- Xia, L.; Shentu, B.Q.; Weng, Z.X. A Kinetic Study of the Thermal Degradation of Nylon-6/Attapulgite Nanocomposites. J. Macromol. Sci. Part B 2015, 54, 851–861. [Google Scholar] [CrossRef]
- Zhang, X.X.; Li, Y.K.; Sun, Z.Y. Acceleration of crystal transformation from crystal form II to form I in Polybutene-1 induced by nanoparticles. Polymer 2018, 150, 119–129. [Google Scholar] [CrossRef]
- de Souza, G.P.M.; de Melo Morgado, G.F.; Albers, A.P.F.; Quinteiro, E.; Passador, F.R. Influence of surface modification of attapulgite (ATP) with aminosilane (3-aminopropyl) triethoxysilane for the preparation of LLDPE/ATP nanocomposites. J. Polym. Res. 2022, 29, 1–13. [Google Scholar]
- Hawley, G. Hawley’s Condensed Chemical Dictionary. Soil Sci. 1943, 14, 592–593. [Google Scholar]
- Silva, S.H.F.D.; Santos, P.S.B.D.; Silva, D.T.D.; Briones, R.; Gatto, D.A.; Labidi, J. Kraft Lignin-Based Polyols by Microwave: Optimizing Reaction Conditions. J. Wood Chem. Technol. 2017, 37, 343–358. [Google Scholar] [CrossRef]
- Alfonso, G.C.; Azzurri, F.; Castellano, M. Analysis of calorimetric curves detected during the polymorphic transformation of isotactic polybutene-1. J. Therm. Anal. Calorim. 2001, 66, 197–207. [Google Scholar] [CrossRef]
- Wang, B.; Yang, D.; Zhang, H.R.; Huang, C.; Xiong, L.; Luo, J.X.; Chen, D. Preparation of Esterified Bacterial Cellulose for Improved Mechanical Properties and the Microstructure of Isotactic Polypropylene/Bacterial Cellulose Composites. Polymers 2016, 8, 129. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Lei, Y.; Xu, D.D.; Liu, F.H.; Li, J.W.; Sun, A.H.; Guo, J.J.; Xu, G.J. Improved mechanical and thermal insulation properties of monolithic attapulgite nanofiber/silica aerogel composites dried at ambient pressure. J. Sol. Gel Sci. Technol. 2017, 82, 702–711. [Google Scholar] [CrossRef]
- Destino, J.F.; Dudukovic, N.A.; Johnson, M.A.; Nguyen, D.T.; Yee, T.D.; Egan, G.C.; Sawvel, A.M.; Steele, W.A.; Baumann, T.F.; Duoss, E.B.; et al. 3D Printed Optical Quality Silica and Silica-Titania Glasses from Sol-Gel Feedstocks. Adv. Mater. Technol. 2018, 6, 1700323. [Google Scholar] [CrossRef]
- Wang, B.; Nie, K.; Xue, X.R.; Lin, F.H.; Li, X.Y.; Xue, Y.B.; Luo, J. Preparation of Maleic Anhydride Grafted Polybutene and Its Application in Isotactic Polybutene-1/Microcrystalline Cellulose Composites. Polymers 2018, 10, 393. [Google Scholar] [CrossRef]
- Zhuravlev, E.; Schmelzer, J.W.P.; Abyzov, A.S.; Fokin, V.M.; Androsch, R.; Schick, C. Experimental Test of Tammann’s Nuclei Development Approach in Crystallization of Macromolecules. Cryst. Growth Des. 2015, 15, 786–798. [Google Scholar] [CrossRef]
- Qiao, Y.N.; Wang, H.; Men, Y.F. Retardance of Form II to Form I Transition in Polybutene-1 at Late Stage: A Proposal of a New Mechanism. Macromolecules 2018, 51, 2232–2239. [Google Scholar] [CrossRef]
- Dou, J.Y.; Liu, Z.P. Crystallization behavior of poly(ethylene terephthalate)/pyrrolidinium ionic liquid. Polym. Int. 2013, 62, 1698–1710. [Google Scholar] [CrossRef]














| Sample | iPB/g | ATP/g | FATP/g | MAFT/g |
|---|---|---|---|---|
| iPB | 1500 | 0 | 0 | 0 |
| iPB + 1% ATP | 1500 | 15 | 0 | 0 |
| iPB + 3% ATP | 1500 | 45 | 0 | 0 |
| iPB + 5% ATP | 1500 | 75 | 0 | 0 |
| iPB + 7% ATP | 1500 | 105 | 0 | 0 |
| iPB + 1% FATP | 1500 | 0 | 15 | 0 |
| iPB + 3% FATP | 1500 | 0 | 45 | 0 |
| iPB + 5% FATP | 1500 | 0 | 75 | 0 |
| iPB + 7% FATP | 1500 | 0 | 105 | 0 |
| iPB + 5% MAFT | 1500 | 0 | 0 | 75 |
| iPB | iPB + 5% MAFT | iPB + 5% ATP | iPB + 5% FATP | |
|---|---|---|---|---|
| n | 1.282 | 0.969 | 1.041 | 1.068 |
| k | 0.026 | 0.029 | 0.021 | 0.043 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, S.-D.; Zhang, M.; Lin, F.-H.; Li, X.-Y.; Zhao, Y.-Y.; Zhang, Y.-L.; Gao, Y.-F.; Luo, J.; Chen, X.-D.; Wang, B. Attapulgite Structure Reset to Accelerate the Crystal Transformation of Isotactic Polybutene. Polymers 2022, 14, 3820. https://doi.org/10.3390/polym14183820
Mao S-D, Zhang M, Lin F-H, Li X-Y, Zhao Y-Y, Zhang Y-L, Gao Y-F, Luo J, Chen X-D, Wang B. Attapulgite Structure Reset to Accelerate the Crystal Transformation of Isotactic Polybutene. Polymers. 2022; 14(18):3820. https://doi.org/10.3390/polym14183820
Chicago/Turabian StyleMao, Shuang-Dan, Mi Zhang, Fu-Hua Lin, Xiang-Yang Li, Yu-Ying Zhao, Yan-Li Zhang, Yi-Fan Gao, Jun Luo, Xin-De Chen, and Bo Wang. 2022. "Attapulgite Structure Reset to Accelerate the Crystal Transformation of Isotactic Polybutene" Polymers 14, no. 18: 3820. https://doi.org/10.3390/polym14183820
APA StyleMao, S. -D., Zhang, M., Lin, F. -H., Li, X. -Y., Zhao, Y. -Y., Zhang, Y. -L., Gao, Y. -F., Luo, J., Chen, X. -D., & Wang, B. (2022). Attapulgite Structure Reset to Accelerate the Crystal Transformation of Isotactic Polybutene. Polymers, 14(18), 3820. https://doi.org/10.3390/polym14183820