Tribological and Rheological Properties of Poly(vinyl alcohol)-Gellan Gum Composite Hydrogels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of PVA Methacrylates and GG Methacrylates
2.2. Fabrication of Composite PVA/GG Hydrogels
2.3. Rheological Tests
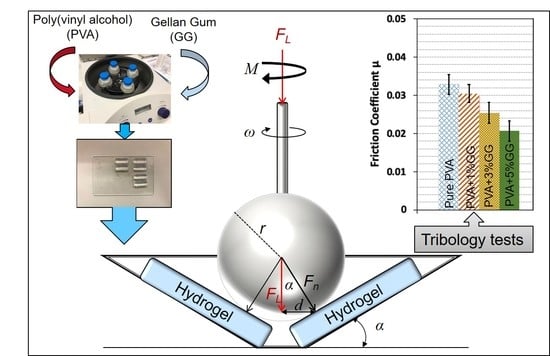
2.4. Tribology Tests
3. Results and Discussions
3.1. Rheological Properties
3.2. Tribological Properties
3.2.1. Effect of Gellan Gum Content on the Friction of PVA/GG Hydrogels
3.2.2. The Effect of Load on Friction
3.2.3. The Effect of Sliding Velocity on the Friction Coefficient
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wichterle, O.; Lim, D. Hydrophilic gels for biological use. Nature 1960, 185, 117–118. [Google Scholar] [CrossRef]
- Wang, F.; Wen, Y.; Bai, T. The composite hydrogels of polyvinyl alcohol–gellan gum-Ca2+ with improved network structure and mechanical property. Mater. Sci. Eng. C 2016, 69, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Rodrigues, J.; Tomas, H. Injectable and biodegradable hydrogels: Gelation, biodegradation and biomedical applications. Chem. Soc. Rev. 2012, 41, 2193–2221. [Google Scholar] [CrossRef] [PubMed]
- Benya, P.D.; Shaffer, J.D. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell 1982, 30, 215–224. [Google Scholar] [CrossRef]
- Nguyen, B.V.; Wang, Q.G.; Kuiper, N.J.; El Haj, A.J.; Thomas, C.R.; Zhang, Z. Biomechanical properties of single chondrocytes and chondrons determined by micromanipulation and finite-element modelling. J. R. Soc. Interface 2010, 7, 1723–1733. [Google Scholar] [CrossRef]
- Holloway, J.L.; Lowman, A.M.; Palmese, G.R. Mechanical evaluation of poly (vinyl alcohol)-based fibrous composites as biomaterials for meniscal tissue replacement. Acta Biomater. 2010, 6, 4716–4724. [Google Scholar] [CrossRef]
- Spiller, K.L.; Maher, S.A.; Lowman, A.M. Hydrogels for the repair of articular cartilage defects. Tissue Eng. Part B Rev. 2011, 17, 281–299. [Google Scholar] [CrossRef]
- Yamaoka, H.; Asato, H.; Ogasawara, T.; Nishizawa, S.; Takahashi, T.; Nakatsuka, T.; Koshima, I.; Nakamura, K.; Kawaguchi, H.; Chung, U.I.; et al. Cartilage tissue engineering using human auricular chondrocytes embedded in different hydrogel materials. J. Biomed. Mater. Res. Part A 2006, 78, 1–11. [Google Scholar] [CrossRef]
- Passaretti, D.; Silverman, R.P.; Huang, W.; Kirchhoff, C.H.; Ashiku, S.; Randolph, M.A.; Yaremchuk, M.J. Cultured chondrocytes produce injectable tissue-engineered cartilage in hydrogel polymer. Tissue Eng. 2001, 7, 805–815. [Google Scholar] [CrossRef]
- Wu, S.; Hua, M.; Alsaid, Y.; Du, Y.; Ma, Y.; Zhao, Y.; Lo, C.Y.; Wang, C.; Wu, D.; Yao, B.; et al. Poly (Vinyl Alcohol) Hydrogels with Broad-Range Tunable Mechanical Properties via the Hofmeister Effect. Adv. Mater. 2021, 33, 2007829. [Google Scholar] [CrossRef]
- Chaudhuri, O.; Gu, L.; Klumpers, D.; Darnell, M.; Bencherif, S.A.; Weaver, J.C.; Huebsch, N.; Lee, H.P.; Lippens, E.; Duda, G.N.; et al. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat. Mater. 2016, 15, 326–334. [Google Scholar] [CrossRef]
- Gorbacheva, S.N.; Yadykova, A.Y.; Ilyin, S.O. Rheological and tribological properties of low-temperature greases based on cellulose acetate butyrate gel. Carbohydr. Polym. 2021, 272, 118509. [Google Scholar] [CrossRef]
- Walker, P.S.; Dowson, D.; Longfield, M.D.; Wright, V.E.R.N.A. “Boosted lubrication” in synovial joints by fluid entrapment and enrichment. Ann. Rheum. Dis. 1968, 27, 512. [Google Scholar] [CrossRef] [PubMed]
- Ateshian, G.A. The role of interstitial fluid pressurization in articular cartilage lubrication. J. Biomech. 2009, 42, 1163–1176. [Google Scholar] [CrossRef]
- Moore, A.C.; Burris, D.L. Tribological rehydration of cartilage and its potential role in preserving joint health. Osteoarthr. Cartil. 2017, 25, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Klein, J. Hydration lubrication. Friction 2013, 1, 1–23. [Google Scholar] [CrossRef]
- Jabbarzadeh, A. Tribological Properties of Interfacial Molecular Films. In Encyclopedia of Interfacial Chemistry Surface Science and Electrochemistry; Wandelt, K., Ed.; Elsevier: Cambridge, MA, USA, 2018; Volume 4, pp. 864–874. [Google Scholar]
- Accardi, M.A.; Dini, D.; Cann, P.M. Experimental and numerical investigation of the behaviour of articular cartilage under shear loading—Interstitial fluid pressurization and lubrication mechanisms. Tribol. Int. 2011, 44, 565–578. [Google Scholar] [CrossRef]
- Porte, E.; Cann, P.; Masen, M. Fluid load support does not explain tribological performance of PVA hydrogels. J. Mech. Behav. Biomed. Mater. 2019, 90, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.P. Friction and lubrication of hydrogels—Its richness and complexity. Soft Matter 2006, 2, 544–552. [Google Scholar] [CrossRef]
- Gong, J.; Iwasaki, Y.; Osada, Y.; Kurihara, K.; Hamai, Y. Friction of gels. 3. Friction on solid surfaces. J. Phys. Chem. B 1999, 103, 6001–6006. [Google Scholar] [CrossRef]
- Koyano, T.; Minoura, N.; Nagura, M.; Kobayashi, K.I. Attachment and growth of cultured fibroblast cells on PVA/chitosan-blended hydrogels. J. Biomed. Mater. Res. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. 1998, 39, 486–490. [Google Scholar] [CrossRef]
- Kumar, A.; Han, S.S. PVA-based hydrogels for tissue engineering: A review. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 159–182. [Google Scholar] [CrossRef]
- Wu, G.; Zhao, C.H.; Wang, C.; Zhang, W. The effect of preparation methods on tribological properties of PVA-H/HA composites. Iran. Polym. J. 2008, 17, 811–819. [Google Scholar]
- Ferris, C.J.; Gilmore, K.J.; Wallace, G.G. Modified gellan gum hydrogels for tissue engineering applications. Soft Matter 2013, 9, 3705–3711. [Google Scholar] [CrossRef]
- Silva-Correia, J.; Oliveira, J.M.; Caridade, S.G.; Oliveira, J.T.; Sousa, R.A.; Mano, J.F.; Reis, R.L. Gellan gum-based hydrogels for intervertebral disc tissue-engineering applications. J. Tissue Eng. Regen. Med. 2010, 5, e97–e107. [Google Scholar] [CrossRef] [PubMed]
- Nafea, E.; Poole-Warren, L.; Martens, P. Bioactivity of permselective PVA hydrogels with mixed ECM analogues. J. Biomed. Mater. Res. Part A 2015, 103, 3727–3735. [Google Scholar] [CrossRef]
- Xu, Z.; Li, Z.; Jiang, S.; Bratlie, K.M. Chemically modified gellan gum hydrogels with tunable properties for use as tissue engineering scaffolds. ACS Omega 2018, 3, 6998–7007. [Google Scholar] [CrossRef]
- Moreira-Izurieta, F.; Jabbarzadeh, A. Tribological studies in cartilaginous tissue of lamb synovial joints lubricated by distilled water and interstitial-fluid-like solution. Tribol. Ind. 2017, 39, 319. [Google Scholar] [CrossRef]
- Instruction Manual for Tribology Measuring Cell T-PTD200; Anton Paar GmbH: Graz, Austria, 2014.
- Rennie, A.C.; Dickrell, P.L.; Sawyer, W.G. Friction coefficient of soft contact lenses: Measurements and modeling. Tribol. Lett. 2005, 18, 499–504. [Google Scholar] [CrossRef]
- Wu, G.; Wang, C.; Zhang, W. The factors of speeds and loads on the tribological properties of PVA-H/HA composites. J. Appl. Polym. Sci. 2007, 106, 3908–3914. [Google Scholar] [CrossRef]
- Meyers, M.A.; Chawla, K.K. Mechanical Behavior of Materials; Cambridge University Press: Cambridge, UK, 2008; p. 125. [Google Scholar]
- Mezger, T.G. Applied Rheology: With Joe Flow on Rheology Road; Anton Paar: Graz, Austria, 2015. [Google Scholar]
- Dai, L.; Liu, X.; Tong, Z. Critical behavior at sol–gel transition in gellan gum aqueous solutions with KCl and CaCl2 of different concentrations. Carbohydr. Polym. 2010, 81, 207–212. [Google Scholar] [CrossRef]
- Mahajan, H.S.; Gattani, S.G. Gellan gum based microparticles of metoclopromide hydrochloride for intranasal delivery: Development and evaluation. Chem. Pharm. Bull. 2009, 57, 388–392. [Google Scholar] [CrossRef] [PubMed]
- Covert, R.J.; Ott, R.D.; Ku, D.N. Friction characteristics of a potential articular cartilage biomaterial. Wear 2003, 255, 1064–1068. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, Y.; Dai, S.-C.; Lim, K.; Ramaswamy, Y.; Jabbarzadeh, A. Tribological and Rheological Properties of Poly(vinyl alcohol)-Gellan Gum Composite Hydrogels. Polymers 2022, 14, 3830. https://doi.org/10.3390/polym14183830
Feng Y, Dai S-C, Lim K, Ramaswamy Y, Jabbarzadeh A. Tribological and Rheological Properties of Poly(vinyl alcohol)-Gellan Gum Composite Hydrogels. Polymers. 2022; 14(18):3830. https://doi.org/10.3390/polym14183830
Chicago/Turabian StyleFeng, Yang, Shao-Cong Dai, Khoon Lim, Yogambha Ramaswamy, and Ahmad Jabbarzadeh. 2022. "Tribological and Rheological Properties of Poly(vinyl alcohol)-Gellan Gum Composite Hydrogels" Polymers 14, no. 18: 3830. https://doi.org/10.3390/polym14183830
APA StyleFeng, Y., Dai, S. -C., Lim, K., Ramaswamy, Y., & Jabbarzadeh, A. (2022). Tribological and Rheological Properties of Poly(vinyl alcohol)-Gellan Gum Composite Hydrogels. Polymers, 14(18), 3830. https://doi.org/10.3390/polym14183830