Improving the Heat and Ablation Resistance of Silicone Rubber Composites by Incorporating Hollow Microspheres
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Sample Preparation
2.3. Characterization
3. Results and Discussion
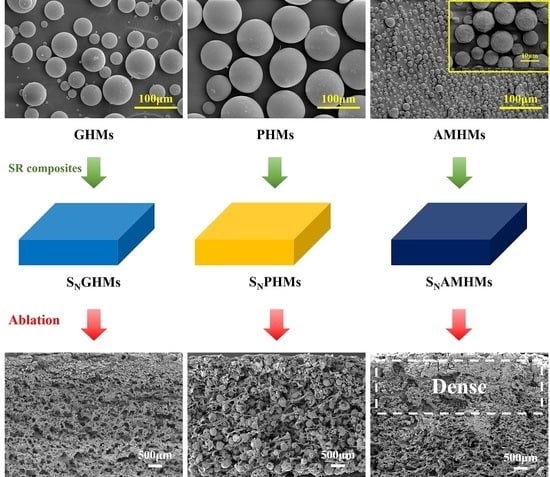
3.1. Structure Analysis of the Microspheres and the Corresponding Composites

3.2. Mechanical Properties

3.3. Thermogravimetric Analysis of Different Hollow Microspheres

3.4. Thermogravimetric Analysis of the SR Composites with Different the Contents of Hollow Microspheres


3.5. Ablation Properties

3.6. Thermal Insulation Performance

3.7. Microscopic Morphology

4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Natali, M.; Kenny, J.M.; Torre, L. Science and technology of polymeric ablative materials for thermal protection systems and propulsion devices: A review. Prog. Mater. Sci. 2016, 84, 192–275. [Google Scholar] [CrossRef]
- Kumar, C.V.; Kandasubramanian, B. Advances in ablative composites of carbon based materials: A review. Ind. Eng. Chem. Res. 2019, 58, 22663–22701. [Google Scholar] [CrossRef]
- Natali, M.; Puri, I.; Kenny, J.M.; Torre, L.; Rallini, M. Microstructure and ablation behavior of an affordable and reliable nanostructured Phenolic Impregnated Carbon Ablator (PICA). Polym. Degrad. Stab. 2017, 141, 84–96. [Google Scholar] [CrossRef]
- George, K.; Panda, B.P.; Mohanty, S.; Nayak, S.K. Recent developments in elastomeric heat shielding materials for solid rocket motor casing application for future perspective. Polym. Adv. Technol. 2018, 29, 8–21. [Google Scholar] [CrossRef]
- Yin, R.; Cheng, H.; Hong, C.; Zhang, X. Synthesis and characterization of novel phenolic resin/silicone hybrid aerogel composites with enhanced thermal, mechanical and ablative properties. Compos. Part. A Appl. S. 2017, 101, 500–510. [Google Scholar] [CrossRef]
- Xiao, J.; Chen, J.; Zhou, H.; Zhang, Q. Study of several organic resin coatings as anti-ablation coatings for supersonic craft control actuator. Mater. S. Eng. A 2007, 452, 23–30. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, H.; Zhang, X.; Yan, L.; Ling, Y.; Zou, H.; Chen, Y.; Liang, M. Effect of mesophase pitch incorporation on the ablation behavior and mechanism of phenolic composites. Ind. Eng. Chem. Res. 2022, 61, 4612–4624. [Google Scholar] [CrossRef]
- Mirzapour, A.; Asadollahi, M.H.; Baghshaei, S.; Akbari, M. Effect of nanosilica on the microstructure, thermal properties and bending strength of nanosilica modified carbon fiber/phenolic nanocomposite. Compos. Part. A Appl. S. 2014, 63, 159–167. [Google Scholar] [CrossRef]
- Qu, H.; Wang, L.; Hui, K.; Bian, C.; Li, H.; Guan, Y.; Luan, T.; Yan, N. Enhancing thermal insulation of EPDM ablators via constructing alternating planar architectures. Polymers 2022, 14, 1570. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, S.; Wang, L.; Lin, H.; Liu, W.; Wu, D.; Wu, Z. Synergetic effects of Kevlar fibers and polyimide powder fillers on performances of Poly(diaryloxyphosphazene) based elastomeric heat shielding materials. Compos. Sci. Technol. 2022, 223, 109439. [Google Scholar] [CrossRef]
- Chen, J.; He, J.; Liu, L.; He, Y.; Huang, X. Effect of hybrid cross-linked polyphosphazene microspheres on ablative and mechanical properties of ethylene propylene diene monomer. J. Appl. Polym. Sci. 2021, 138, 51348. [Google Scholar] [CrossRef]
- Smitha Alex, A.; Rajeev, R.S.; Krishnaraj, K.; Sreenivas, N.; Manu, S.K.; Gouri, C.; Sekkar, V. Thermal protection characteristics of polydimethylsiloxane-organoclay nanocomposite. Polym. Degrad. Stab. 2017, 144, 281–291. [Google Scholar] [CrossRef]
- Liu, H.; Zhu, G.; Zhang, C. Promoted ablation resistance of polydimethylsiloxane via crosslinking with multi-ethoxy POSS. Compos. Part. B Eng. 2020, 190, 107901. [Google Scholar] [CrossRef]
- Yan, L.; Cai, Y.; Luo, Y.; Sun, T.; Zhou, S.; Chen, Y.; Zou, H.; Liang, M.; Yang, Y. Ablation response behavior under different heat flux environments for liquid silicone rubber composites. ACS Appl. Polym. Mater. 2021, 3, 5632–5641. [Google Scholar] [CrossRef]
- Arabgol, F.; Kokabi, M.; Bahramian, A.R. Ablation behavior of organoclay-NBR insulator: Modeling and experimental. Fire. Mater. 2018, 42, 859–872. [Google Scholar] [CrossRef]
- Natali, M.; Rallini, M.; Kenny, J.; Torre, L. Effect of wollastonite on the ablation resistance of EPDM based elastomeric heat shielding materials for solid rocket motors. Polym. Degrad. Stab. 2016, 130, 47–57. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, S.; Liu, W.; An, G.; Wu, Z.; Wu, D.; Jin, R. Nitrile butadiene rubber-based heat-shielding insulations for solid rocket motors. High. Perform. Polym. 2014, 27, 153–160. [Google Scholar] [CrossRef]
- Yang, D.; Zhang, W.; Jiang, B.; Guo, Y. Silicone rubber ablative composites improved with zirconium carbide or zirconia. Compos. Part. A Appl. S 2013, 44, 70–77. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, Z.; Yan, L.; Chen, Y.; Liang, M.; Zou, H. Ordered graphitized ceramic layer induced by liquid crystal epoxy resin in silicone rubber composites with enhanced ablation resistance performance. Mater. Chem. Phys. 2021, 270, 124823. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, J.; Zhu, H.; Ali, S.; Ma, H.; Zhang, L.; Wu, D.; Wu, Z. Evaluation of poly(diaryloxyphosphazene) elastomer for heat shielding insulations and morphology of charred layers. High. Perform. Polym. 2016, 29, 450–457. [Google Scholar] [CrossRef]
- Polmanteer, K. Current perspectives on silicone rubber technology. Rubber. Chem. Technol. 1981, 54, 1051–1080. [Google Scholar] [CrossRef]
- Song, J.; Huang, Z.; Qin, Y.; Wang, H.; Shi, M. Effects of zirconium silicide on the vulcanization, mechanical and ablation resistance properties of ceramifiable silicone rubber composites. Polymers 2020, 12, 496. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Su, J.; Yin, Z.; Li, Y.; Zhi, Y.; Gao, J. Ablation and mechanical investigation of heat vulcanizing silicone rubber (HVSR) composite containing carbon fibers. J. Polym. Eng. 2017, 37, 521–528. [Google Scholar] [CrossRef]
- Zou, Z.; Qin, Y.; Tian, Q.; Huang, Z.; Zhao, Z. The influence of zirconia fibre on ablative composite materials. Plast. Rubber Compos. 2019, 48, 185–190. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, C.; Li, Y.; Yin, Z.; Zhi, Y.; Gao, J. Ablative properties and mechanisms of hot vulcanised silicone rubber (HVSR) composites containing different fillers. Plast. Rubber Compos. 2016, 45, 430–435. [Google Scholar] [CrossRef]
- Soo Kim, E.; Lee, T.H.; Shin, S.H.; Yoon, J.-S. Effect of incorporation of carbon fiber and silicon carbide powder into silicone rubber on the ablation and mechanical properties of the silicone rubber-based ablation material. J. Appl. Polym. Sci. 2011, 120, 831–838. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, F.; Dai, J.; Huang, Z. Effect of functionalization of graphene nanoplatelets on the mechanical and thermal properties of silicone rubber composites. Materials 2016, 9, 92. [Google Scholar] [CrossRef]
- Li, P.; Duan, H.; Liu, Y.; Chi, W.; Huang, Q. Multi-walled carbon nanotubes as secondary fibre fillers for property improvement of short carbon fibre-reinforced silicone rubber. B Mater. Sci. 2019, 42, 4. [Google Scholar] [CrossRef]
- Kumar, V.; Alam, M.N.; Manikkavel, A.; Song, M.; Lee, D.J.; Park, S.S. Silicone rubber composites reinforced by carbon nanofillers and their hybrids for various applications: A review. Polymers 2021, 13, 2322. [Google Scholar] [CrossRef]
- Xi, K.; Li, J.; Wang, Y.; Guo, M.; Li, K. Thermal insulation and char layer mechanical properties of a novel ethylene propylene diene monomer composite reinforced with carbon nanotubes coated via chemical vapour deposition. Compos. Sci. Technol. 2021, 201, 108537. [Google Scholar] [CrossRef]
- Rallini, M.; Puri, I.; Torre, L.; Natali, M. Thermal and ablation properties of EPDM based heat shielding materials modified with density reducer fillers. Compos. Part A Appl. S. 2018, 112, 71–80. [Google Scholar] [CrossRef]
- Yan, L.; Zhang, H.; Zhou, S.; Zou, H.; Chen, Y.; Liang, M. Improving ablation properties of liquid silicone rubber composites by in situ construction of rich porous char layer. J. Appl. Polym. Sci. 2020, 138, 50030. [Google Scholar] [CrossRef]
- Lin, J.; Ma, C. Thermal degradation of phenolic resin/silica hybrid ceramers. Polym. Degrad. Stab. 2000, 69, 229–235. [Google Scholar] [CrossRef]
- Trick, K.A.; Saliba, T.E. Mechanisms of the pyrolysis of phenolic resin in a carbon/phenolic composite. Carbon 1995, 33, 1509–1515. [Google Scholar] [CrossRef]
- He, C.; Li, B.; Ren, Y.; Lu, W.; Zeng, Y.; He, W.; Feng, A. How the Crosslinking Agent Influences the Thermal Stability of RTV Phenyl Silicone Rubber. Materials 2018, 12, 88. [Google Scholar] [CrossRef]
- Hamdani, S.; Longuet, C.; Perrin, D.; Lopez-cuesta, J.-M.; Ganachaud, F. Flame retardancy of silicone-based materials. Polym. Degrad. Stab. 2009, 94, 465–495. [Google Scholar] [CrossRef]
- Li, Z.; Lin, W.; Moon, K.-S.; Wilkins, S.J.; Yao, Y.; Watkins, K.; Morato, L.; Wong, C. Metal catalyst residues in carbon nanotubes decrease the thermal stability of carbon nanotube/silicone composites. Carbon 2011, 49, 4138–4148. [Google Scholar] [CrossRef]
- Liu, T.; Zeng, X.; Lai, X.; Li, H.; Wang, Y. Remarkable improvement of organic-to-inorganic conversion of silicone rubber at elevated temperature through platinum-nitrogen catalytic system. Polym. Degrad. Stab. 2020, 171, 109026. [Google Scholar] [CrossRef]
- Chen, D.; Liu, Y.; Huang, C. Synergistic effect between POSS and fumed silica on thermal stabilities and mechanical properties of room temperature vulcanized (RTV) silicone rubbers. Polym. Degrad. Stab. 2012, 97, 308–315. [Google Scholar] [CrossRef]
- Guo, M.; Li, J.; Wang, Y. Effects of carbon nanotubes on char structure and heat transfer in ethylene propylene diene monomer composites at high temperature. Compos. Sci. Technol. 2021, 211, 108852. [Google Scholar] [CrossRef]
| Samples | Ingredients in Phr | |||||||
|---|---|---|---|---|---|---|---|---|
| E-LSR | SiO2 | PBO | GHM | PHM | AMHM | KH550 | DBTDL | |
| Pure | 100 | 0 | 0 | 0 | 0 | 0 | 6 | 0.8 |
| S0 | 100 | 5 | 6 | / | / | / | 6 | 0.8 |
| S10/20/30/40GHM | 100 | 5 | 6 | 10/20/ 30/40 | / | / | 6 | 0.8 |
| S10/20/30/40PHM | 100 | 5 | 6 | / | 10/20/ 30/40 | / | 6 | 0.8 |
| S10/20/30/40AMHM | 100 | 5 | 6 | / | / | 10/20/ 30/40 | 6 | 0.8 |
| Samples | Tm (°C) |
|---|---|
| S0 | −44.51 |
| S30GHM | −44.06 |
| S30PHM | −43.60 |
| S30AMHM | −43.98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, J.; Yan, L.; Zhang, H.; Zhou, S.; Xia, S.; Zou, H. Improving the Heat and Ablation Resistance of Silicone Rubber Composites by Incorporating Hollow Microspheres. Polymers 2022, 14, 3846. https://doi.org/10.3390/polym14183846
Tian J, Yan L, Zhang H, Zhou S, Xia S, Zou H. Improving the Heat and Ablation Resistance of Silicone Rubber Composites by Incorporating Hollow Microspheres. Polymers. 2022; 14(18):3846. https://doi.org/10.3390/polym14183846
Chicago/Turabian StyleTian, Jinfeng, Liwei Yan, Hao Zhang, Shengtai Zhou, Shuang Xia, and Huawei Zou. 2022. "Improving the Heat and Ablation Resistance of Silicone Rubber Composites by Incorporating Hollow Microspheres" Polymers 14, no. 18: 3846. https://doi.org/10.3390/polym14183846
APA StyleTian, J., Yan, L., Zhang, H., Zhou, S., Xia, S., & Zou, H. (2022). Improving the Heat and Ablation Resistance of Silicone Rubber Composites by Incorporating Hollow Microspheres. Polymers, 14(18), 3846. https://doi.org/10.3390/polym14183846