Synthesis of Highly Conductive Poly(3-hexylthiophene) by Chemical Oxidative Polymerization Using Surfactant Templates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
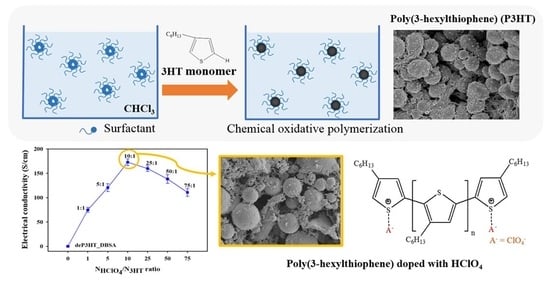
2.2. Synthesis of Poly(3-hexylthiophene)
2.3. De-Doping/Doping Process
2.4. Characterizations
3. Results and Discussion
3.1. Structural Confirmation of the Synthesized P3HT
3.2. UV-Vis Spectroscopy
3.3. X-ray Diffraction
3.4. X-ray Photoelectron Spectrometer
3.5. Thermal Gravimetric Analysis
3.6. Morphology
3.7. Electrical Conductivity
3.8. De-Doping/Doping Process
3.9. The Correlation of Electrical Conductivity and Doping Level
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Liu, A.; Han, Y.; Li, T. Sensors based on conductive polymers and their composites: A review. Polym. Int. 2020, 69, 7–17. [Google Scholar] [CrossRef]
- Krathumkhet, N.; Imae, T.; Paradee, N. Electrically controlled transdermal ibuprofen delivery consisting of pectin-bacterial cellulose/polypyrrole hydrogel composites. Cellulose 2021, 28, 11451–11463. [Google Scholar] [CrossRef]
- He, X.; Shi, G. Electrochemical actuator based on monolithic polypyrrole–TiO2 nanoparticle composite film. Sens. Actuators B Chem. 2006, 115, 488–493. [Google Scholar] [CrossRef]
- Lin, K.Y.; Hu, L.W.; Chen, K.L.; Siao, M.D.; Ji, W.F.; Yang, C.C.; Yeh, J.M.; Chiu, K.C. Characterization of polyaniline synthesized from chemical oxidative polymerization at various polymerization temperatures. Eur. Polym. J. 2017, 88, 311–319. [Google Scholar] [CrossRef]
- Palaniappan, S.; John, A. Polyaniline materials by emulsion polymerization pathway. Prog. Polym. Sci. 2008, 33, 732–758. [Google Scholar] [CrossRef]
- Zhang, X.; Meng, X.; Wang, Q.; Qin, B.; Jin, L.E.; Cao, Q. Preparation and electrochemical investigation of polyaniline nanowires for high performance supercapacitor. Mater. Lett. 2018, 217, 312–315. [Google Scholar] [CrossRef]
- Barros, R.A.; Areias, M.C.C.; Azevedo, W.M. Conducting polymer photopolymerization mechanism: The role of nitrate ions (NO3−). Synth. Met. 2010, 160, 61–64. [Google Scholar] [CrossRef]
- Freitas, T.V.; Sousa, E.A.; Fuzari, G.C.; Arlindo, E.P.S. Different morphologies of polyaniline nanostructures synthesized by interfacial polymerization. Mater. Lett. 2018, 224, 42–45. [Google Scholar] [CrossRef]
- Meng, H.; Perepichka, D.F.; Wudl, F. Facile solid-state synthesis of highly conducting poly(ethylenedioxythiophene). Angew. Chem. Int. Ed. 2003, 42, 658–661. [Google Scholar] [CrossRef]
- Kumar, D.; Sharma, R.C. Advances in conductive polymers. Eur. Polym. J. 1998, 34, 1053–1060. [Google Scholar] [CrossRef]
- Hoshina, Y.; Zaragoza-Contreras, E.A.; Farnood, R.; Kobayashi, T. Nanosized polypyrrole affected by surfactant agitation for emulsion polymerization. Polym. Bull. 2011, 68, 1689–1705. [Google Scholar] [CrossRef]
- Bhadra, S.; Khastgir, D.; Singha, N.K.; Lee, J.H. Progress in preparation, processing and applications of polyaniline. Prog. Polym. Sci. 2009, 34, 783–810. [Google Scholar] [CrossRef]
- Paradee, N.; Sirivat, A. Synthesis of poly(3,4-ethylenedioxythiophene) nanoparticles via chemical oxidation polymerization. Polym. Int. 2014, 63, 106–113. [Google Scholar] [CrossRef]
- Kulkarni, M.V.; Viswanath, A.K.; Khanna, P.K. Synthesis and humidity sensing properties of conducting poly(N-methyl aniline) doped with different acids. Sens. Actuators B Chem. 2006, 115, 140–149. [Google Scholar] [CrossRef]
- Manaf, A.; Hafizah, M.A.E.; Riyadi, A.F.; Andreas. Electrical conductivity of polyaniline (PANI) assisted by anionic surfactant through emulsion polymerization technique. J. Phys. Conf. Ser. 2019, 1153, 012067. [Google Scholar] [CrossRef]
- Direksilp, C.; Sirivat, A. Synthesis and characterization of hollow-sphered poly(N-methyaniline) for enhanced electrical conductivity based on the anionic surfactant templates and doping. Polymers 2020, 12, 1023. [Google Scholar] [CrossRef]
- Hai, T.A.P.; Sugimoto, R. Surface functionalization of cellulose with poly(3-hexylthiophene) via novel oxidative polymerization. Carbohydr. Polym. 2018, 179, 221–227. [Google Scholar] [CrossRef]
- Sharma, T.; Singhal, R.; Vishnoi, R.; Lakshmi, G.B.V.S.; Chand, S.; Avasthi, D.K.; Kanjilal, A.; Biswas, S.K. Ion irradiation induced modifications of P3HT: A donor material for organic photovoltaic devices. Vacuum 2017, 135, 73–85. [Google Scholar] [CrossRef]
- Tang, K.; Huang, L.; Lim, J.; Zaveri, T.; Azoulay, J.D.; Guo, S. Chemical doping of well-dispersed P3HT thin-film nanowire networks. ACS Appl. Polym. Mater. 2019, 1, 2943–2950. [Google Scholar] [CrossRef]
- Zhang, S.; Yan, H.; Yeh, J.-M.; Shi, X.; Zhang, P. Electroactive composite of FeCl3-doped P3HT/PLGA with adjustable electrical conductivity for potential application in neural tissue engineering. Macromol. Biosci. 2019, 19, 1900147. [Google Scholar] [CrossRef]
- Lakdusinghe, M.; Abbaszadeh, M.; Mishra, S.; Sengottuvelu, D.; Wijayapala, R.; Zhang, S.; Benasco, A.R.; Gu, X.; Morgan, S.E.; Wipf, D.O.; et al. Nanoscale self-assembly of poly(3-hexylthiophene) assisted by a low-molecular-weight gelator toward large-scale fabrication of electrically conductive networks. ACS Appl. Nano Mater. 2021, 4, 8003–8014. [Google Scholar] [CrossRef]
- Pecher, J.; Mecking, S. Nanoparticles of conjugated polymers. Chem. Rev. 2010, 110, 6260–6279. [Google Scholar] [CrossRef]
- Khanh, T.S.T.; Trung, T.Q.; Giang, L.T.T.; Nguyen, T.Q.; Lam, N.D.; Dinh, N.N. Ammonia Gas Sensing Characteristic of P3HT-rGO-MWCNT Composite Films. Appl. Sci. 2021, 11, 6675. [Google Scholar] [CrossRef]
- Tanusorn, N.; Thummarungsan, N.; Sangwan, W.; Lerdwijitjarud, W.; Sirivat, A. Influence of carrageenan molecular structures on electromechanical behaviours of poly(3-hexylthiophene)/carrageenan conductive hydrogels. Int. J. Biol. Macromol. 2018, 118, 2098–2107. [Google Scholar] [CrossRef]
- Kleinschmidt, A.T.; Root, S.E.; Lipomi, D.J. Poly(3-hexylthiophene) (P3HT): Fruit fly or outlier in organic solar cell research. J. Mater. Chem. A 2017, 5, 11396–11400. [Google Scholar] [CrossRef]
- Lai, C.H.; Ashby, D.S.; Lin, T.C.; Lau, J.; Dawson, A.; Tolbert, S.H.; Dunn, D.S. Application of poly(3-hexylthiophene-2,5-diyl) as a protective coating for high rate cathode materials. Chem. Mater. 2018, 30, 2589–2599. [Google Scholar] [CrossRef]
- Fukumoto, H.; Omori, Y.; Yamamoto, T. Effects of solvent and temperature on regioregularity of poly(3-hexylthiophene-2,5-diyl) prepared by chemical oxidative polymerization. Polym. Int. 2012, 45, 462–465. [Google Scholar] [CrossRef]
- Duong, D.T.; Wang, C.; Antono, E.; Toney, M.F.; Salleo, A. The chemical and structural origin of efficient p-type doping in P3HT. Org. Electron. 2013, 14, 1330–1336. [Google Scholar] [CrossRef]
- Fuentes-Perez, M.; Nicho, M.E.; Sotelo-Lerma, M.; Fuentes-Rios, J.L.; Castrello-Uribe, J.; Leon-Silva, U.; Hernandez-Guzman, F.; Garcia-Carvajal, S. Influence of the FeO9OH) nanoparticles concentration in the in-situ synthesis of P3HT. Eur. Polym. J. 2018, 99, 172–179. [Google Scholar] [CrossRef]
- Giddings, L.D.; Olesik, S.V. A study of AOT reverse micelles in liquids at ambient and high pressure. Langmuir 1994, 10, 2877–2883. [Google Scholar] [CrossRef]
- Sharma, G.; Bhogal, S.; Naushad, M.; Inamuddin; Kumar, A.; Stadler, F.J. Microwave assisted fabrication of La/Cu/Zr/carbon dots trimetallic nanocomposites with their adsorptional vs photocatalytic efficiency for remediation of persistent organic pollutants. J. Photochem. Photobiol. 2017, 347, 235–243. [Google Scholar] [CrossRef]
- Ansari, M.A.; Mohiuddin, S.; Kandemirli, F.; Malik, M.I. Synthesis and characterization of poly(3-hexylthiophene): Improvement of regioregularity and energy band gap. RSC Adv. 2018, 8, 8319–8328. [Google Scholar] [CrossRef]
- Rodrigues, A.; Castro, M.C.R.; Farinha, A.S.F.; Oliveira, M.; Tomé, J.P.C.; Machado, A.V.; Raposo, M.M.M.; Hilliou, L.; Bernardo, G. Thermal stability of P3HT and P3HT:PCBM blends in the molten state. Polym. Test. 2013, 32, 1192–1201. [Google Scholar] [CrossRef]
- Alhreb, B.M.; Almasri, K.; Alhariri, S. Fabrication and characterization of poly(3-hexylthiophene) (P3HT) sensor in two techniques (dip-coating and spin-coating) and sensitivity compared for various vapors. Int. J. Chemtech. Res. 2014, 6, 3690–3696. [Google Scholar]
- Hammed, W.A.; Rahman, M.S.; Mahmud, H.N.M.E.; Yahya, R.; Sulaiman, K. Processable dodecylbenzene sulfonic acid (DBSA) doped poly(N-vinyl carbazole)-poly(pyrrole) for optoelectronic applications. Des. Monomers Polym. 2016, 20, 368–377. [Google Scholar] [CrossRef]
- Phasuksom, K.; Sirivat, A. Synthesis of nano-sized polyindole via emulsion polymerization and doping. Synth. Met. 2016, 219, 142–153. [Google Scholar] [CrossRef]
- Mao, H.; Liu, X.; Qian, X.; An, X. Preparation and dedoping-resistant effect of self-doped polyaniline/cellulose fibers (SPANI/CF) hybrid. Cellulose 2015, 22, 1–10. [Google Scholar] [CrossRef]
- Suresh, S.; Mohideen, N.N.; Vinitha, G.; Kumar, R.M. Synthesis, growth, structural, optical and electrical properties of novel organic single crystal: P-toluidinium salicylate. Mod. Electron. Mater. 2018, 4, 103–111. [Google Scholar] [CrossRef]
- Sivaraman, P.; Mishra, S.P.; Bhattacharrya, A.R.; Thakur, A.; Shashidhara, K.; Samui, A.B. Effect of regioregularity on specific capacitance of poly(3-hexylthiophene). Electrochim. Acta 2012, 69, 134–138. [Google Scholar] [CrossRef]
- Du, Y.; Cai, K.F.; Shen, S.Z.; Casey, P.S. Preparation and characterization of graphene nanosheets/poly(3-hexylthiophene) thermoelectric composite materials. Synth. Met. 2012, 162, 2102–2106. [Google Scholar] [CrossRef]
- Usharani, S.; Rajendran, V. Morphologically controlled synthesis, structural and optical properties of CeO2/SnO2 nanocomposites. J. Sci. Adv. Mater. Dev. 2017, 2, 333–339. [Google Scholar]
- Hrostea, L.; Girtan, M.; Mallet, R.; Leontie, L. Optical and morphological properties of P3HT and P3HT: PCBM thin films used in photovoltaic applications. IOP Conf. Ser. Mater. Sci. Eng. 2018, 374, 012015. [Google Scholar] [CrossRef] [Green Version]
- Kar, P. Doping in Conjugated Polymers, 1st ed.; Scrivener Publishing LLC: Beverly, MA, USA, 2013; pp. 7–9. [Google Scholar]
- Enengl, C.; Enengl, S.; Pluczyk, S.; Havlicek, M.; Lapkowski, M.; Neugebauer, H.; Ehrenfreund, E. Doping-induced absorption bands in P3HT: Polarons and bipolarons. ChemPhysChem 2016, 17, 3836–3844. [Google Scholar] [CrossRef]
- Tan, B.; Li, Y.; Palacios, M.F.; Therrien, J.; Sobkowicz, M.J. Effect of surfactant conjugation on structure and properties of poly(3-hexylthiophene) colloids and field effect transistors. Colloids Surf. A Physicochem. Eng. 2016, 488, 7–14. [Google Scholar] [CrossRef]
- Golczak, S.; Kanciurzewska, A.; Fahlman, M.; Langer, K.; Langer, J.J. Comparative XPS surface study of polyaniline thin films. Solid State Ion. 2008, 179, 2234–2239. [Google Scholar] [CrossRef]
- Yemata, T.A.; Zheng, Y.; Kyaw, A.K.K.; Wang, X.; Song, J.; Chin, W.S.; Xu, J. Modulation of the doping level of PEDOT: PSS film by treatment with hydrazine to improve the Seebeck coefficient. RSC Adv. 2020, 10, 1786–1792. [Google Scholar] [CrossRef]
- Rehmen, J.; Zuber, K.; Modarresi, M.; Kim, D.; Charrault, E.; Jannasch, P.; Zozoulenko, I.; Evans, D.; Karlsson, C. Structural control of charge storage capacity to achieve 100% doping in vapor phase-polymerized PEDOT/tosylate. ACS Omega 2019, 4, 21818–21826. [Google Scholar] [CrossRef]
- Lyutov, V.; Kabanova, V.; Gribkova, O.; Nekrasov, A.; Tsakova, V. Electrochemically obtained polysulfonates doped poly(3,4-ethylenedioxythiophene) films—effects of the dopant’s chain flexibility and molecular weight studied by electrochemical, microgravimetric and XPS methods. Polymers 2021, 13, 2438. [Google Scholar] [CrossRef]
- Tungkavet, T.; Seetapan, N.; Pattavarakorn, D.; Sirivat, A. Improvements of electromechanical properties of gelatin hydrogels by blending with nanowire polypyrrole: Effects of electric field and temperature. Polym. Int. 2012, 61, 825–833. [Google Scholar] [CrossRef]
- Jang, J.H.; Ha, J.; Kim, S. Fabrication of polyaniline nanoparticles using microemulsion polymerization. Macromol. Res. 2007, 15, 154–159. [Google Scholar] [CrossRef]
- Zheng, H.; Jiang, Y.; Xu, J.; Yang, Y. The characteristic properties of PEDOT nano-particle based on reversed micelle method. Sci. China Technol. Sci. 2010, 53, 2355–2362. [Google Scholar] [CrossRef]
- Alveroglu, E. Doping effect of dodecyl benzene sulphonic acid in poly(3-hexylthiophene)-P3HT-films. J. Mol. Struct. 2015, 1086, 86–92. [Google Scholar] [CrossRef]
- Karim, M.R. Synthesis and characterizations of poly(3-hexylthiophene) and modified carbon nanotube composite. J. Nanomater. 2012, 2012, 174353. [Google Scholar] [CrossRef]
- Sakunpongpitiporn, P.; Phasuksom, K.; Paradee, N.; Sirivat, A. Facile synthesis of highly conductive PEDOT: PSS via surfactant templates. RSC Adv. 2019, 9, 6363–6378. [Google Scholar] [CrossRef] [Green Version]










| Synthesis Method | Surfactant Type | Particle Size (nm) | Particle Shape | Electrical Conductivity (S cm−1) | Sample Code | Ref. |
|---|---|---|---|---|---|---|
| Chemical oxidative polymerization | - | 157 ± 33 | Irregular | 3.12 ± 0.44 | P3HT at 6 h | - |
| - | 141 ± 21 | Irregular | 8.09 ± 0.12 | P3HT at 12 h | - | |
| - | 280 ± 62 | Agglomerated | 3.04 ± 0.43 | P3HT at 18 h | - | |
| - | 311 ± 69 | Agglomerated | 2.33 ± 0.20 | P3HT at 24 h | - | |
| - | 650 ± 197 | Irregular | 0.24 ± 0.05 | 3HT:FeCl3 = 1:2.5 | - | |
| - | 141 ± 21 | Irregular | 8.09 ± 0.12 | 3HT:FeCl3 = 1:3 | - | |
| - | 191 ± 48 | Agglomerated | 1.23 ± 0.36 | 3HT:FeCl3 = 1:3.5 | - | |
| - | 347 ± 139 | Agglomerated | 0.49 ± 0.12 | 3HT:FeCl3 = 1:4 | - | |
| Emulsion polymerization | DBSA | 244 ± 58 | Sphere cluster and fiber | 5.82 ± 1.38 | P3HT_DBSA 2 CMC | - |
| 542 ± 139 | Spherical | 7.05 ± 0.74 | P3HT_DBSA 4 CMC | - | ||
| 1526 ± 227 | Grape shape | 16.21 ± 1.55 | P3HT_DBSA 6 CMC | - | ||
| 672 ± 192 | Sphere cluster | 10.41 ± 2.27 | P3HT_DBSA 8 CMC | - | ||
| 516 ± 131 | Sphere cluster and fiber | 3.17 ± 0.13 | P3HT_DBSA 10 CMC | - | ||
| PTSA | 356 ± 65 | Irregular | 1.07 ± 0.05 | P3HT_PTSA 2 CMC | - | |
| 316 ±82 | Irregular | 3.13 ± 0.30 | P3HT_PTSA 4 CMC | - | ||
| 254 ± 58 | Coral reef shape | 6.33 ± 0.27 | P3HT_PTSA 6 CMC | - | ||
| 364 ± 118 | Fused spheres and fiber | 0.82 ± 0.04 | P3HT_PTSA 8 CMC | - | ||
| 414 ± 70 | Fused spheres and fiber | 0.04 ± 0.01 | P3HT_PTSA 10 CMC | - | ||
| SDS | 247 ± 38 | Spherical | 0.41 ± 0.03 | P3HT_SDS 2 CMC | - | |
| 234 ± 48 | Spherical | 2.80 ± 0.18 | P3HT_SDS 4 CMC | - | ||
| 300 ± 49 | Spherical | 0.76 ± 0.08 | P3HT_SDS 6 CMC | - | ||
| 498 ± 98 | Sphere cluster | 0.58 ± 0.12 | P3HT_SDS 8 CMC | - | ||
| 817 ± 54 | Sphere cluster | 0.35 ± 0.02 | P3HT_SDS 10 CMC | - | ||
| AOT | 348 ± 92 | Spherical | 0.06 ± 0.01 | P3HT_AOT 2 CMC | - | |
| 335 ± 97 | Spherical and fiber | 0.11 ± 0.03 | P3HT_AOT 4 CMC | - | ||
| 269 ± 98 | Spherical and fiber | 0.65 ± 0.05 | P3HT_AOT 6 CMC | - | ||
| 278 ± 73 | Spherical and fiber | 0.57 ± 0.02 | P3HT_AOT 8 CMC | - | ||
| 291 ± 53 | Spherical and fiber | 0.37 ± 0.03 | P3HT_AOT 10 CMC | - | ||
| De-doping with NH4OH | - | 1545 ± 861 | Spherical | 2.01 × 10−5 | deP3HT_DBSA | - |
| Doped with HClO4 | - | 1223 ± 718 | Spherical | 74.81 ± 5.22 | dP3HT_DBSA (1:1) | - |
| - | 1232 ± 463 | Spherical | 120.56 ± 8.10 | dP3HT_DBSA (5:1) | - | |
| - | 1110 ± 494 | Spherical | 172.74 ± 7.02 | dP3HT_DBSA (10:1) | - | |
| - | 1389 ± 873 | Spherical | 159.68 ± 6.11 | dP3HT_DBSA (25:1) | - | |
| - | 1170 ± 654 | Spherical | 138.24 ± 9.25 | dP3HT_DBSA (50:1) | - | |
| - | 1530 ± 827 | Spherical | 110.70 ± 8.01 | dP3HT_DBSA (75:1) | - | |
| In situ polymerization | - | - | Agglomerated | 2.30 × 10−5 | P3HT | [48] |
| - | - | Tubular | 0.71 | P3HT_MWNT | [48] | |
| Chemical oxidative polymerization | - | - | Agglomerated | 1.20 | P3HT_GNs | [34] |
| - | - | - | 3.00 | P3HT | [21] | |
| - | - | - | 1.82 ± 0.22 | P3HT doped with F4TCNQ | [22] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kesornsit, S.; Direksilp, C.; Phasuksom, K.; Thummarungsan, N.; Sakunpongpitiporn, P.; Rotjanasuworapong, K.; Sirivat, A.; Niamlang, S. Synthesis of Highly Conductive Poly(3-hexylthiophene) by Chemical Oxidative Polymerization Using Surfactant Templates. Polymers 2022, 14, 3860. https://doi.org/10.3390/polym14183860
Kesornsit S, Direksilp C, Phasuksom K, Thummarungsan N, Sakunpongpitiporn P, Rotjanasuworapong K, Sirivat A, Niamlang S. Synthesis of Highly Conductive Poly(3-hexylthiophene) by Chemical Oxidative Polymerization Using Surfactant Templates. Polymers. 2022; 14(18):3860. https://doi.org/10.3390/polym14183860
Chicago/Turabian StyleKesornsit, Sanhanut, Chatrawee Direksilp, Katesara Phasuksom, Natlita Thummarungsan, Phimchanok Sakunpongpitiporn, Kornkanok Rotjanasuworapong, Anuvat Sirivat, and Sumonman Niamlang. 2022. "Synthesis of Highly Conductive Poly(3-hexylthiophene) by Chemical Oxidative Polymerization Using Surfactant Templates" Polymers 14, no. 18: 3860. https://doi.org/10.3390/polym14183860
APA StyleKesornsit, S., Direksilp, C., Phasuksom, K., Thummarungsan, N., Sakunpongpitiporn, P., Rotjanasuworapong, K., Sirivat, A., & Niamlang, S. (2022). Synthesis of Highly Conductive Poly(3-hexylthiophene) by Chemical Oxidative Polymerization Using Surfactant Templates. Polymers, 14(18), 3860. https://doi.org/10.3390/polym14183860