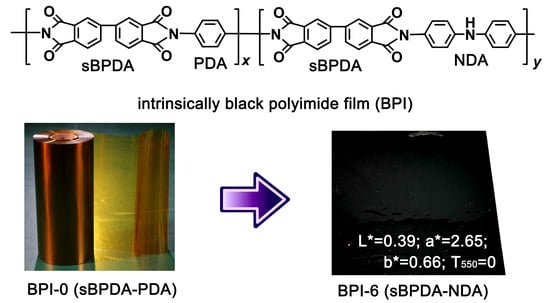
Preparation and Properties of Intrinsically Black Polyimide Films with CIE Lab Color Parameters Close to Zero and High Thermal Stability for Potential Applications in Flexible Printed Circuit Boards
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Measurements
2.3. Preparation of PI films
3. Results and Discussion
3.1. PI Films Preparation
3.2. Optical Properties
3.3. Thermal Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gouzman, I.; Grossman, E.; Verker, R.; Atar, N.; Bolker, A.; Eliaz, N. Advances in polyimide-based materials for space applications. Adv. Mater. 2019, 31, 1807738. [Google Scholar] [CrossRef] [PubMed]
- Baig, U.; Faizan, M.; Dastageer, M.A. Polyimide based super-wettable membrane/materials for high performance oil/water mixture and emulsion separation: A review. Adv. Colloid Inter Sci. 2021, 297, 102525. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Sui, F.; Miao, Y.; Liu, G.; Li, C.; Dong, W.; Cui, J.; Liu, T.; Wu, J.; Yang, C. Polyimide separators for rechargeable batteries. J. Energy Chem. 2021, 58, 170–197. [Google Scholar] [CrossRef]
- Park, S.; Chang, H.Y.; Rahimi, S.; Lee, A.L.; Tao, L.; Akinwande, D. Transparent nanoscale polyimide gate dielectric for highly flexible electronics. Adv. Electron. Mater. 2018, 4, 1700043. [Google Scholar] [CrossRef]
- Shi, S.; Yao, L.; Ma, P.; Jiao, Y.; Zheng, X.; Ning, D.; Chen, M.; Sui, F.; Liu, H.; Yang, C.; et al. Recent progress in the high-temperature-resistant PI substrate with low CTE for CIGS thin-film solar cells. Mater. Today Energy 2021, 20, 100640. [Google Scholar] [CrossRef]
- Ni, H.J.; Liu, J.G.; Wang, Z.H.; Yang, S.Y. A review on colorless and optically transparent polyimide films: Chemsitry, process and engineering applications. J. Ind. Eng. Chem. 2015, 28, 16–27. [Google Scholar] [CrossRef]
- Yi, C.; Li, W.; Shi, S.; He, K.; Ma, P.; Chen, M.; Yang, C. High-temperature-resistant and colorless polyimide: Preparations, properties, and applications. Sol. Energy 2020, 195, 340–354. [Google Scholar] [CrossRef]
- Yan, X.; Dai, F.; Ke, Z.; Yan, K.; Chen, C.; Qian, G.; Li, H. Synthesis of colorless polyimides with high Tg from asymmetric twisted benzimidazole diamines. Eur. Polym. J. 2022, 164, 110975. [Google Scholar] [CrossRef]
- Ozawa, H.; Ishiguro, E.; Kyoya, Y.; Kikuchi, Y.; Matsumoto, T. Colorless polyimides derived from an alicyclic tetracarboxylic dianhydride, CpODA. Polymers 2021, 13, 2824. [Google Scholar] [CrossRef]
- Miao, J.; Hu, X.; Wang, X.; Meng, X.; Wang, Z.; Yan, J. Colorless polyimides derived from adamantine-containing diamines. Polym. Chem. 2020, 11, 6009–6016. [Google Scholar] [CrossRef]
- Tapaswi, P.K.; Ha, C.S. Recent trends on transparent colorless polyimides with balanced thermal and optical properties: Design and synthesis. Macromol. Chem. Phys. 2019, 220, 1800313. [Google Scholar] [CrossRef]
- Chen, L.; Yu, H.; Dirican, M.; Fang, D.; Tian, Y.; Yan, C.; Xie, J.; Jia, D.; Liu, H.; Wang, J.; et al. Highly transparent and colorless nanocellulose/polyimide substrates with enhanced thermal and mechanical properties for flexible OLED displays. Adv. Mater. Interf. 2022, 7, 2000928. [Google Scholar] [CrossRef]
- Qian, M.; Liu, G.; Zhou, B.; Xuan, X.Y.; Niu, Y.P.; Gong, S.Q. Atomic oxygen durable ultra-black polyimide nanocomposite films in solar spectrum. Polym. Degrad. Stab. 2020, 175, 109133. [Google Scholar] [CrossRef]
- Shivakumar, R.; Bolker, A.; Tsang, S.H.; Atar, N.; Verker, R.; Gouzman, I.; Hala, M.; Moshe, N.; Jones, A.; Grossman, E.; et al. POSS enhanced 3D graphene-Polyimide film for atomic oxygen endurance in Low Earth Orbit space environment. Polymer 2020, 191, 122270. [Google Scholar] [CrossRef]
- Lin, J.S.; Chiu, H.T. Preparation and properties of conductive polyimide films. J. Polym. Res. 2002, 9, 189–194. [Google Scholar] [CrossRef]
- Ando, S.; Matsuura, T.; Sasaki, S. Coloration of aromatic polyimides and electronic properties of their source materials. Polym. J. 1997, 29, 69–76. [Google Scholar] [CrossRef]
- Xue, P.; Wang, J.; Bao, Y.; Li, Q.; Wu, C. Synergistic effect between carbon black nanoparticles and polyimide on refractive indices of polyimide/carbon black nanocomposites. N. J. Chem. 2012, 36, 903–910. [Google Scholar] [CrossRef]
- Kim, J.; Kwon, J.; Lee, D.; Kim, M.; Han, H. Heat dissipation properties of polyimide nanocomposite films. Korean J. Chem. Eng. 2016, 33, 3245–3250. [Google Scholar] [CrossRef]
- Kwon, J.; Kim, J.; Lee, J.; Han, P.; Park, D.; Han, H. Fabrication of polyimide composite films based on carbon black for high-temperature resistance. Polym. Compos. 2014, 35, 2214–2220. [Google Scholar] [CrossRef]
- Murugaraj, P.; Mainwaring, D.E.; Mora-Huertas, N. Electromechanical response of semiconducting carbon–polyimide nanocomposite thin films. Compos. Sci. Technol. 2009, 69, 2454–2459. [Google Scholar] [CrossRef]
- Jiang, X.; Bin, Y.; Matsuo, M. Electrical and mechanical properties of polyimide–carbon nanotubes composites fabricated by in situ polymerization. Polymer 2005, 46, 7418–7424. [Google Scholar] [CrossRef]
- Norian, K.H. Electrical properties of carbon black-polyimide thick films. Thin Soli Films 1989, 168, 169–174. [Google Scholar] [CrossRef]
- Xue, P.; Bao, Y.; Li, Q.; Wu, C. Impact of modification of carbon black on morphology and performance of polyimide/carbon black hybrid composites. Phys. Chem. Chem. Phys. 2010, 12, 11342–11350. [Google Scholar] [CrossRef]
- Liu, T.; Zheng, F.; Ding, T.; Zhang, S.; Lu, Q. Design and synthesis of a novel quinoxaline diamine and its polyimides with high-Tg and red color. Polymer 2019, 179, 121612. [Google Scholar] [CrossRef]
- Liu, T.; Zheng, F.; Ma, X.; Ding, T.; Chen, S.; Jiang, W.; Zhang, S.; Lu, Q. High heat-resistant polyimide films containing quinoxaline moiety for flexible substrate applications. Polymer 2020, 209, 122963. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, S.; Zheng, F.; Lu, Q. Intrinsically black polyimide with retained insulation and thermal properties: A black anthraquinone derivative capable of linear copolymerization. Macromolecules 2021, 54, 9307–9318. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, Y.; Liu, J.; Zhi, X.; Huangfu, M.; Jiang, G.; Wu, X.; Zhang, X. Molecular design, synthesis and characterization of intrinsically black polyimide films with high thermal stability and good electrical properties. J. Polym. Res. 2019, 26, 171. [Google Scholar] [CrossRef]
- Tan, Y.Y.; Zhang, Y.; Jiang, G.L.; Zhi, X.X.; Xiao, X.; Wu, L.; Jia, Y.J.; Liu, J.G. Zhang X M. Preparation and properties of inherently black polyimide films with extremely low coefficients of thermal expansion and potential applications for black flexible copper clad laminates. Polymers 2020, 12, 576. [Google Scholar] [CrossRef]
- Wakita, J.; Sekino, H.; Sakai, K.; Urano, Y.; Ando, S. Molecular design, synthesis, and properties oh highly fluorescent polyimides. J. Phys. Chem. B 2009, 113, 15212–15224. [Google Scholar] [CrossRef]
- Chen, C.J.; Yen, H.J.; Hu, Y.C.; Liou, G.S. Novel programmable functional polyimides: Preparation, mechanism of CT induced memory, and ambipolar electrochromic behavior. J. Mater. Chem. C 2013, 1, 7623–7634. [Google Scholar] [CrossRef]
- Lei, H.; Li, X.; Wang, J.; Song, Y.; Tian, G.; Huang, M.; Wu, D. DFT and molecular dynamic simulation for the dielectric property analysis of polyimides. Chem. Phys. Lett. 2022, 786, 139131. [Google Scholar] [CrossRef]
- Unsal, E.; Cakmak, M. Real-time characterization of physical changes in polyimide film formation: From casting to imidization. Macromolecules 2013, 46, 8616–8627. [Google Scholar] [CrossRef]
- Chen, W.; Chen, W.; Zhang, B.; Yang, S.; Liu, C. Thermal imidization process of polyimide film: Interplay between solvent evaporation and imidization. Polymer 2017, 109, 205–215. [Google Scholar] [CrossRef]











| Samples | Mn a (×104 g/mol) | Mw a (×104 g/mol) | PDI a |
|---|---|---|---|
| PAA-0 | 2.73 | 4.19 | 1.53 |
| PAA-1 | 2.75 | 6.36 | 2.31 |
| PAA-2 | 2.80 | 6.68 | 2.38 |
| PAA-3 | 2.90 | 7.12 | 2.46 |
| PAA-4 | 2.93 | 7.43 | 2.54 |
| PAA-5 | 2.93 | 7.44 | 2.54 |
| PAA-6 | 3.04 | 9.66 | 3.18 |
| Samples | λcut a (nm) | T550 a (%) | T650 a (%) | T760 a (%) | L* a | a* a | b* a | WI a | Haze (%) |
|---|---|---|---|---|---|---|---|---|---|
| BPI-1 | 415 | 9.7 | 40.2 | 63.4 | 38.45 | 35.62 | 65.74 | 3.16 | 1.64 |
| BPI-2 | 499 | 5.0 | 32.7 | 59.5 | 27.38 | 37.70 | 47.13 | 5.57 | 0.40 |
| BPI-3 | 525 | 1.3 | 23.4 | 53.5 | 23.78 | 37.36 | 40.97 | 5.75 | 0.66 |
| BPI-4 | 529 | 0.6 | 14.2 | 43.3 | 13.07 | 31.86 | 22.51 | 4.72 | 0 |
| BPI-5 | 563 | 0 | 5.6 | 29.5 | 4.27 | 22.51 | 7.34 | 1.39 | 0 |
| BPI-6 | 572 | 0 | 1.4 | 22.5 | 0.39 | 2.65 | 0.66 | 0.35 | 1.83 |
| PI-ref1 | 407 | 70.4 | 74.6 | 80.9 | 88.65 | −9.35 | 79.41 | 14.45 | 0.68 |
| PI-ref2 | 592 (555) b | 0 (0.4) | 3.6 (34.8) | 36.8 (72.4) | 2.20 (23.36) | 11.99 (46.43) | 3.33 (40.26) | 1.41 (1.76) | 0 (0) |
| PI | T5% a (°C) | T10% a (°C) | Tmax a (°C) | Rw750 a (%) | Tg b (°C) | CTE c (×10−6/K) |
|---|---|---|---|---|---|---|
| BPI-0 | ND d | ND | ND | ND | ND | 12.3 |
| BPI-1 | 625.9 | 646.1 | 662.0 | 69.2 | ND | 15.3 |
| BPI-2 | 617.8 | 639.3 | 655.3 | 70.1 | 349.5 | 20.9 |
| BPI-3 | 609.6 | 632.8 | 649.5 | 69.9 | 347.6 | 26.6 |
| BPI-4 | 609.3 | 632.2 | 646.8 | 71.1 | 365.5 | 28.9 |
| BPI-5 | 602.4 | 625.3 | 642.3 | 71.6 | 349.0 | 32.2 |
| BPI-6 | 591.3 | 619.1 | 631.3 | 72.7 | 351.7 | 34.8 |
| PI-ref1 | 581.0 | 594.8 | 605.1 | 61.6 | 418.8 | 29.5 |
| PI-ref2 | 515.7 | 559.6 | 591.3 | 59.6 | 431.6 | 18.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, X.; Zhang, Y.; Liu, Y.; Yang, C.; Dai, S.; Wang, X.; Liu, J. Preparation and Properties of Intrinsically Black Polyimide Films with CIE Lab Color Parameters Close to Zero and High Thermal Stability for Potential Applications in Flexible Printed Circuit Boards. Polymers 2022, 14, 3881. https://doi.org/10.3390/polym14183881
Ren X, Zhang Y, Liu Y, Yang C, Dai S, Wang X, Liu J. Preparation and Properties of Intrinsically Black Polyimide Films with CIE Lab Color Parameters Close to Zero and High Thermal Stability for Potential Applications in Flexible Printed Circuit Boards. Polymers. 2022; 14(18):3881. https://doi.org/10.3390/polym14183881
Chicago/Turabian StyleRen, Xi, Yan Zhang, Yuang Liu, Changxu Yang, Shengwei Dai, Xiaolei Wang, and Jingang Liu. 2022. "Preparation and Properties of Intrinsically Black Polyimide Films with CIE Lab Color Parameters Close to Zero and High Thermal Stability for Potential Applications in Flexible Printed Circuit Boards" Polymers 14, no. 18: 3881. https://doi.org/10.3390/polym14183881
APA StyleRen, X., Zhang, Y., Liu, Y., Yang, C., Dai, S., Wang, X., & Liu, J. (2022). Preparation and Properties of Intrinsically Black Polyimide Films with CIE Lab Color Parameters Close to Zero and High Thermal Stability for Potential Applications in Flexible Printed Circuit Boards. Polymers, 14(18), 3881. https://doi.org/10.3390/polym14183881