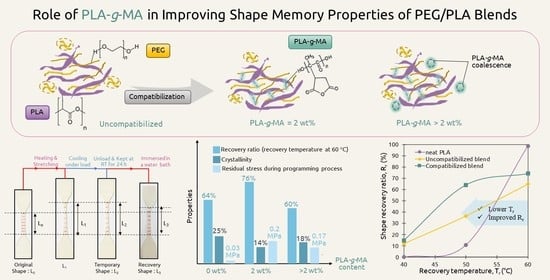
Role of Maleic Anhydride-Grafted Poly(lactic acid) in Improving Shape Memory Properties of Thermoresponsive Poly(ethylene glycol) and Poly(lactic acid) Blends
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of PLA-g-MA and PEG/PLA Blends
2.3. Characterizations of PLA-g-MA
2.4. Characterizations of PEG/PLA Blends
2.4.1. Tensile Testing
2.4.2. Thermal Characteristics
2.4.3. Morphology
2.4.4. Shape Memory Behaviors
2.4.5. Stress Relaxation Test
2.4.6. Microstructure Evaluation
3. Results and Discussion
3.1. Characterizations of PLA-g-MA
3.2. Tensile Properties of PLA and PEG/PLA Blends with Various PLA-g-MA Contents
3.3. Thermal Behaviors of PLA and PEG/PLA Blends with Various PLA-g-MA Contents
3.4. Morphologies of PLA and PEG/PLA Blends with Various PLA-g-MA Contents
3.5. Shape Memory Behaviors of PLA and PEG/PLA Blends
3.5.1. Shape Memory Behaviors of the PLA and PEG/PLA Blends with Various PLA-g-MA Contents
3.5.2. Shape Memory Behaviors of the PLA and PEG/PLA Blends at Various Recovery Temperatures
3.6. Stress Relaxation of the PEG/PLA Blends with Various PLA-g-MA Contents
3.7. Microstructures of PEG/PLA and 2PMA/PEG/PLA Blends during Shape Memory Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, K.; Jia, Y.-G.; Zhao, C.; Zhu, X.X. Multiple and two-way reversible shape memory polymers: Design strategies and applications. Prog. Mater. Sci. 2019, 105, 100572. [Google Scholar] [CrossRef]
- Hager, M.D.; Bode, S.; Weber, C.; Schubert, U.S. Shape memory polymers: Past, present and future developments. Prog. Mater. Sci. 2015, 49–50, 3–33. [Google Scholar] [CrossRef]
- Lendlein, A.; Gould, O.E.C. Reprogrammable recovery and actuation behaviour of shape-memory polymers. Nat. Rev. Mater. 2019, 4, 116–133. [Google Scholar] [CrossRef]
- Yang, J.-M.; Panda, P.K.; Jie, C.J.; Dash, P.; Chang, Y.-H. Poly(vinyl alcohol)/chitosan/sodium alginate composite blended membrane: Preparation, characterization, and water-induced shape memory phenomenon. Polym. Eng. Sci. 2022, 62, 1526–1537. [Google Scholar] [CrossRef]
- Chatterjee, T.; Dey, P.; Nando, G.B.; Naskar, K. Thermo-responsive shape memory polymer blends based on alpha olefin and ethylene propylene diene rubber. Polymer 2015, 78, 180–192. [Google Scholar] [CrossRef]
- Tonndorf, R.; Aibibu, D.; Cherif, C. Thermoresponsive Shape Memory Fibers for Compression Garments. Polymers 2020, 12, 2989. [Google Scholar] [CrossRef] [PubMed]
- Herath, M.; Epaarachchi, J.; Islam, M.; Fang, L.; Leng, J. Light activated shape memory polymers and composites: A review. Eur. Polym. J. 2020, 136, 109912. [Google Scholar] [CrossRef]
- Yao, J.; Liu, X.; Sun, H.; Liu, S.; Jiang, Y.; Yu, B.; Ning, N.; Tian, M.; Zhang, L. Thermoplastic Polyurethane Dielectric Elastomers with High Actuated Strain and Good Mechanical Strength by Introducing Ester Group Grafted Polymethylvinylsiloxane. Ind. Eng. Chem. Res. 2021, 60, 4883–4891. [Google Scholar] [CrossRef]
- Ze, Q.; Kuang, X.; Wu, S.; Wong, J.; Montgomery, S.M.; Zhang, R.; Kovitz, J.M.; Yang, F.; Qi, H.J.; Zhao, R. Magnetic Shape Memory Polymers with Integrated Multifunctional Shape Manipulation. Adv. Mater. 2020, 32, 1906657. [Google Scholar] [CrossRef]
- He, H.; Shi, X.; Chen, W.; Chen, R.; Zhao, C.; Wang, S. Temperature/pH Smart Nanofibers with Excellent Biocompatibility and Their Dual Interactions Stimulus-Responsive Mechanism. J. Agric. Food Chem. 2020, 68, 7425–7433. [Google Scholar] [CrossRef]
- Guo, Y.; Lv, Z.; Huo, Y.; Sun, L.; Chen, S.; Liu, Z.; He, C.; Bi, X.; Fan, X.; You, Z. A biodegradable functional water-responsive shape memory polymer for biomedical applications. J. Mater. Chem. B 2019, 7, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Sun, Y.C.; Ren, J.; Naguib, H.E. Toward the low actuation temperature of flexible shape memory polymer composites with room temperature deformability via induced plasticizing effect. J. Mater. Chem. B 2017, 5, 8845–8853. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, J.; Wu, B.; Zhang, J.; Peng, Y.; Lu, H.; Le, X.; Wei, S.; Chen, T. Supramolecular Assembly of Shape Memory and Actuating Hydrogels for Programmable Shape Transformation. ACS Appl. Mater. Interfaces 2022, 14, 3551–3558. [Google Scholar] [CrossRef] [PubMed]
- Panda, P.K.; Dash, P.; Biswal, A.K.; Chang, Y.-H.; Misra, P.K.; Yang, J.-M. Synthesis and Characterization of Modified Poly(vinyl alcohol) Membrane and Study of Its Enhanced Water-Induced Shape-Memory Behavior. J. Polym. Environ. 2022, 30, 3409–3419. [Google Scholar] [CrossRef]
- Wang, X.; Yang, X.; Xu, C.; Lin, B.; Fu, L. Enhanced, hydrophobic, initial-shape programmable shape-memory composites with a bio-based nano-framework via gradient metal-ligand cross-linking. Compos. Sci. Technol. 2022, 220, 109255. [Google Scholar] [CrossRef]
- Wu, M.; Yang, L.; Shen, Q.; Zheng, Z.; Xu, C. Endeavour to balance mechanical properties and self-healing of nature rubber by increasing covalent crosslinks via a controlled vulcanization. Eur. Polym. J. 2021, 161, 110823. [Google Scholar] [CrossRef]
- Xu, J.; Song, J. Polylactic acid (PLA)-based shape-memory materials for biomedical applications. In Shape Memory Polymers for Biomedical Applications; Yahia, L., Ed.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 197–217. [Google Scholar]
- Li, G.; Zhao, M.; Xu, F.; Yang, B.; Li, X.; Meng, X.; Teng, L.; Sun, F.; Li, Y. Synthesis and Biological Application of Polylactic Acid. Molecules 2020, 25, 5023. [Google Scholar] [CrossRef]
- Kumar Panda, P.; Jebastine, J.; Ramarao, M.; Fairooz, S.; Reddy, C.K.; Nasif, O.; Alfarraj, S.; Manikandan, V.; Jenish, I.; Ganeshan, P. Exploration on Mechanical Behaviours of Hyacinth Fibre Particles Reinforced Polymer Matrix-Based Hybrid Composites for Electronic Applications. Adv. Mater. Sci. Eng. 2021, 2021, 4933450. [Google Scholar] [CrossRef]
- Mehrpouya, M.; Vahabi, H.; Janbaz, S.; Darafsheh, A.; Mazur, T.R.; Ramakrishna, S. 4D printing of shape memory polylactic acid (PLA). Polymer 2021, 230, 124080. [Google Scholar] [CrossRef]
- Barletta, M.; Gisario, A.; Mehrpouya, M. 4D printing of shape memory polylactic acid (PLA) components: Investigating the role of the operational parameters in fused deposition modelling (FDM). J. Manuf. Process. 2021, 61, 473–480. [Google Scholar] [CrossRef]
- Navarro-Baena, I.; Sessini, V.; Dominici, F.; Torre, L.; Kenny, J.M.; Peponi, L. Design of biodegradable blends based on PLA and PCL: From morphological, thermal and mechanical studies to shape memory behavior. Polym. Degrad. Stab. 2016, 132, 97–108. [Google Scholar] [CrossRef]
- Gallos, A.; Crowet, J.-M.; Michely, L.; Raghuwanshi, V.S.; Mention, M.; Langlois, V.; Dauchez, M.; Garnier, G.; Allais, F. Blending Ferulic Acid Derivatives and Polylactic Acid into Biobased and Transparent Elastomeric Materials with Shape Memory Properties. Biomacromolecules 2021, 22, 1568–1578. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.; Yuk, J.; Lee, H.; Kang, S.; Park, H.; Park, S.; Jihoon, S. Lactide-derived ester oligomers for highly compatible poly(lactide) plasticizer produced through an eco-friendly process: Renewable resources, biodegradation, enhanced flexibility, and elastomeric performance. Green Chem. 2021, 23, 7549–7565. [Google Scholar] [CrossRef]
- Jariyasakoolroj, P.; Rojanaton, N.; Jarupan, L. Crystallization behavior of plasticized poly(lactide) film by poly(l-lactic acid)-poly(ethylene glycol)-poly(l-lactic acid) triblock copolymer. Polym. Bull. 2020, 77, 2309–2323. [Google Scholar] [CrossRef]
- Chaos, A.; Sangroniz, A.; Fernández, J.; Río, J.; Iriarte, M.; Sarasua, J.R.; Etxeberria, A. Plasticization of poly(lactide) with poly(ethylene glycol): Low weight plasticizer vs. triblock copolymers. Effect on free volume and barrier properties. J. Appl. Polym. Sci. 2019, 137, 48868. [Google Scholar] [CrossRef]
- Koosomsuan, W.; Yamaguchi, M.; Phinyocheep, P.; Sirisinha, K. High-Strain Shape Memory Behavior of PLA-PEG Multiblock Copolymers and Its Microstructural Origin. J. Polym. Sci. B Polym. Phys. 2019, 57, 241–256. [Google Scholar] [CrossRef]
- Guo, Y.; Ma, J.; Lv, Z.; Zhao, N.; Wang, L.; Li, Q. The effect of plasticizer on the shape memory properties of poly(lactide acid)/poly(ethylene glycol) blends. J. Mater. Res. 2018, 33, 4101–4112. [Google Scholar] [CrossRef]
- Younes, H.; Cohn, D. Phase separation in poly(ethylene glycol)/poly(lactic acid) blends. Eur. Polym. J. 1988, 24, 765–773. [Google Scholar] [CrossRef]
- Wang, B.; Hina, K.; Zou, H.; Zuo, D.; Yi, C. Thermal, crystallization, mechanical and decomposition properties of poly(lactic acid) plasticized with poly(ethylene glycol). J. Vinyl Addit. Technol. 2018, 24, E154–E163. [Google Scholar] [CrossRef]
- Li, F.-J.; Zhang, S.-D.; Liang, J.-Z.; Wang, J.-Z. Effect of polyethylene glycol on the crystallization and impact properties of polylactide-based blends. Polym. Adv. Technol. 2015, 26, 465–475. [Google Scholar] [CrossRef]
- Piontek, A.; Vernaez, O.; Kabasci, S. Compatibilization of Poly(Lactic Acid) (PLA) and Bio-Based Ethylene-Propylene-Diene-Rubber (EPDM) via Reactive Extrusion with Different Coagents. Polymers 2020, 12, 605. [Google Scholar] [CrossRef] [PubMed]
- Luo, F.; Li, J.; Ji, F.; Weng, Y.-X.; Ren, J. Preparation of poly(lactic acid)-based shape memory polymers with low response temperature utilizing composite plasticizers. Polym. Bull. 2022, 79, 4761–4781. [Google Scholar] [CrossRef]
- Raquez, J.-M.; Narayan, R.; Dubois, P. Recent Advances in Reactive Extrusion Processing of Biodegradable Polymer-Based Compositions. Macromol. Mater. Eng. 2008, 293, 447–470. [Google Scholar] [CrossRef]
- Hwang, S.W.; Lee, S.B.; Lee, C.K.; Lee, J.Y.; Shim, J.K.; Selke, S.; Soto-Valdez, H.; Matuana, L.; Rubino, M.; Auras, R. Grafting of maleic anhydride on poly(L-lactic acid). Effects on physical and mechanical properties. Polym. Test. 2012, 31, 333–344. [Google Scholar] [CrossRef]
- Hassouna, F.; Raquez, J.-M.; Addiego, F.; Dubois, P.; Toniazzo, V.; Ruch, D. New approach on the development of plasticized polylactide (PLA): Grafting of poly(ethylene glycol) (PEG) via reactive extrusion. Eur. Polym. J. 2011, 47, 2134–2144. [Google Scholar] [CrossRef]
- Kim, S.K.; Jang, R.; Kim, W.N. Effects of compatibilizer on the mechanical, rheological, and shape memory behaviors of poly(lactic acid) and poly(MnBM) blends. J. Appl. Polym. Sci. 2019, 137, 48591. [Google Scholar] [CrossRef]
- Davachi, S.M.; Kaffashi, B. Preparation and Characterization of Poly L-Lactide/Triclosan Nanoparticles for Specific Antibacterial and Medical Applications. Int. J. Polym. Mater. Polym. Biomater. 2015, 64, 497–508. [Google Scholar] [CrossRef]
- Panda, P.K.; Yang, J.-M.; Chang, Y.-H. Water-induced shape memory behavior of poly(vinyl alcohol) and p-coumaric acid-modified water-soluble chitosan blended membrane. Carbohydr. Polym. 2021, 257, 117633. [Google Scholar] [CrossRef]
- Tang, X.; Liu, C.; Keum, J.; Chen, J.; Dial, B.E.; Wang, Y.; Tsai, W.-Y.; Bras, W.; Saito, T.; Bowland, C.C.; et al. Upcycling of semicrystalline polymers by compatibilization: Mechanism and location of compatibilizers. RSC Adv. 2022, 12, 10886–10894. [Google Scholar] [CrossRef]
- Liu, H.; Xie, T.; Zhang, Y.; Ou, Y.; Yang, G. Crystallization Behaviors of Polypropylene/Polyamide-6 Blends Modified by a Maleated Thermoplastic Elastomer. Polym. J. 2006, 38, 21–30. [Google Scholar] [CrossRef] [Green Version]
- Athanasoulia, I.-G.; Tarantili, P.A. Preparation and characterization of polyethylene glycol/poly(L-lactic acid) blends. Pure Appl. Chem. 2017, 89, 141–152. [Google Scholar] [CrossRef]
- Wang, W.; Jin, Y.; Ping, P.; Chen, X.; Jing, X.; Su, Z. Structure Evolution in Segmented Poly(ester urethane) in Shape-Memory Process. Macromolecules 2010, 43, 2942–2947. [Google Scholar] [CrossRef]
- Tcharkhtchi, A.; Abdallah-Elhirtsi, S.; Ebrahimi, K.; Fitoussi, J.; Shirinbayan, M.; Farzaneh, S. Some New Concepts of Shape Memory Effect of Polymers. Polymers 2014, 6, 1144–1163. [Google Scholar] [CrossRef]
- Kong, D.; Xiao, X. High Cycle-life Shape Memory Polymer at High Temperature. Sci. Rep. 2016, 6, 33610. [Google Scholar] [CrossRef] [PubMed]
- Kallel, A.; Abdallah, A.B.; Gamaoun, F.; Farzaneh, S.; BenDaly, H.; Fitoussi, J.; Tcharkhtchi, A. Driving force for shape memory effect of polymers. J. Polym. Rev. 2021, 28, 308. [Google Scholar] [CrossRef]
- Tian, Y.; Zhu, C.; Gong, J.; Yang, S.; Ma, J.; Xu, J. Lamellae break induced formation of shish-kebab during hot stretching of ultra-high molecular weight polyethylene precursor fibers investigated by in situ small angle X-ray scattering. Polymer 2014, 55, 4299–4306. [Google Scholar] [CrossRef]













| Sample | Tensile Strength [MPa] | Young’s Modulus [GPa] | Elongation at Break [%] |
|---|---|---|---|
| neat PLA | 61.38 ± 1.24 # | 0.70 ± 0.02 # | 12.97 ± 0.92 # |
| PEG/PLA | 40.22 ± 0.60 * | 0.49 ± 0.01 * | 477.01 ± 62.03 * |
| 2PMA/PEG/PLA | 45.04 ± 1.32 *,# | 0.53 ± 0.01 *,# | 475.51 ± 88.87 * |
| 6PMA/PEG/PLA | 48.07 ± 0.90 *,# | 0.55 ± 0.01 *,# | 205.23 ± 73.74 *,# |
| 10PMA/PEG/PLA | 50.15 ± 1.53 *,# | 0.56 ± 0.01 *,# | 74.74 ± 11.78 # |
| Sample | Tg [°C] | Tcc [°C] | −ΔHcc [J/g] | Tm [°C] | ∆Hm [J/g] | Normalized Xc [%] |
|---|---|---|---|---|---|---|
| Unstretched-PLA | 58.3 | 115.5 | 24.9 | 152.3 | 27.4 | 2.7 |
| Unstretched-PEG/PLA | 40.3 | 87.2 | 15.3 | 152.2 | 25.9 | 12.6 |
| Unstretched-2PMA/PEG/PLA | 41.6 | 84.4 | 18.4 | 152.4 | 25.2 | 8.2 |
| Unstretched-6PMA/PEG/PLA | 41.8 | 85.9 | 16.3 | 153.7 | 28.1 | 14.8 |
| Unstretched-10PMA/PEG/PLA | 42.3 | 87.1 | 18.0 | 154.9 | 29.3 | 12.0 |
| Stretched-PLA | 62.2 | 110.7 | 21.3 | 149.9 | 26.9 | 6.1 |
| Stretched-PEG/PLA | 43.8 | 67.5 | 8.6 | 152.8 | 29.5 | 24.8 |
| Stretched-2PMA/PEG/PLA | 41.8 | 71.3 | 16.7 | 154.1 | 27.9 | 13.5 |
| Stretched-6PMA/PEG/PLA | 42.6 | 69.0 | 13.0 | 154.2 | 27.7 | 18.4 |
| Stretched-10PMA/PEG/PLA | 42.8 | 68.8 | 12.4 | 153.2 | 29.4 | 18.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nonkrathok, W.; Trongsatitkul, T.; Suppakarn, N. Role of Maleic Anhydride-Grafted Poly(lactic acid) in Improving Shape Memory Properties of Thermoresponsive Poly(ethylene glycol) and Poly(lactic acid) Blends. Polymers 2022, 14, 3923. https://doi.org/10.3390/polym14183923
Nonkrathok W, Trongsatitkul T, Suppakarn N. Role of Maleic Anhydride-Grafted Poly(lactic acid) in Improving Shape Memory Properties of Thermoresponsive Poly(ethylene glycol) and Poly(lactic acid) Blends. Polymers. 2022; 14(18):3923. https://doi.org/10.3390/polym14183923
Chicago/Turabian StyleNonkrathok, Wasana, Tatiya Trongsatitkul, and Nitinat Suppakarn. 2022. "Role of Maleic Anhydride-Grafted Poly(lactic acid) in Improving Shape Memory Properties of Thermoresponsive Poly(ethylene glycol) and Poly(lactic acid) Blends" Polymers 14, no. 18: 3923. https://doi.org/10.3390/polym14183923
APA StyleNonkrathok, W., Trongsatitkul, T., & Suppakarn, N. (2022). Role of Maleic Anhydride-Grafted Poly(lactic acid) in Improving Shape Memory Properties of Thermoresponsive Poly(ethylene glycol) and Poly(lactic acid) Blends. Polymers, 14(18), 3923. https://doi.org/10.3390/polym14183923