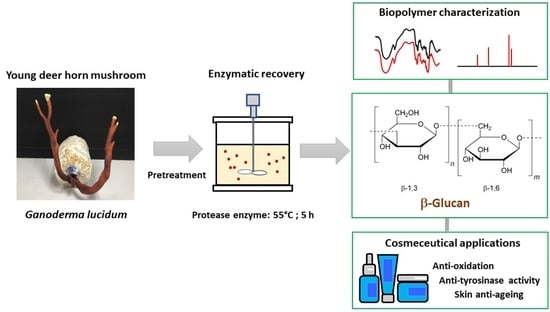
Mushroom β-Glucan Recovered from Antler-Type Fruiting Body of Ganoderma lucidum by Enzymatic Process and Its Potential Biological Activities for Cosmeceutical Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mushroom Pretreatment
2.2. Enzymatic Recovery of BG from Mushroom Fruiting Body
2.3. Determination of BG Content of Antler-Type Fruiting Body G. lucidum
2.4. Analysis of BG Composition
2.5. Fourier Transform Infrared (FTIR) Spectroscopy
2.6. Nuclear Magnetic Resonance Spectroscopy (NMR)
2.7. Analysis of Antioxidant Properties
2.7.1. 1,1-Diphenyl-2-picrylhydrazyl Radical Scavenging (DPPH) Assay
2.7.2. Ferric Reducing Antioxidant Power (FRAP) Assay
2.7.3. 2,2-Azinobis (3-ethylbenzothiazoline-6-sulphonic Acid) (ABTS) Assay
2.8. Analysis of Anti-Tyrosinase Properties
2.9. Analysis of Anti-Hyaluronidase Properties
2.10. Analysis of Anti-Collagenase Properties
2.11. Analysis of Anti-Elastase Properties
2.12. In Vitro Cytotoxicity Test by MTT Assay
2.13. In Vitro Irritation Test by Hen’s Egg Test Chorioallantoic Membrane (HET-CAM) Assay
2.14. Functionality of BG
2.14.1. Water Holding Capacity (WHC)
2.14.2. Water Binding Capacity (WBC)
2.14.3. Swelling Capacity (SC)
2.14.4. Oil Holding Capacity (OHC)
2.15. Statistical Analysis
3. Results and Discussion
3.1. Composition of Antler-Type G. lucidum BG Extract
3.2. Fourier Transform Infrared (FTIR) Spectroscopy of Antler-Type G. lucidum BG
3.3. Nuclear Magnetic Resonance Spectroscopy (NMR) of Antler-Type G. lucidum BG
3.4. Protective Activity
3.5. In Vitro Functional Properties of Antler-Type G. lucidum BG
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Worrasinchai, S.; Suphantharika, M.; Pinjai, S.; Jamnong, P. Glucan prepared from spent brewer’s yeast as a fat replacer in mayonnaise. Food Hydrocoll. 2006, 20, 68–78. [Google Scholar] [CrossRef]
- Abd Razak, D.L.; Jamaluddin, A.; Abd Rashid, N.Y.; Sani, N.A.; Abdul Manan, M. Assessment of cosmeceutical potentials of selected mushroom fruitbody extracts through evaluation of antioxidant, anti-hyaluronidase and anti-tyrosinase activity. J 2020, 3, 329–342. [Google Scholar] [CrossRef]
- Qiu, A.; Wang, Y.; Zhang, G.; Wang, H. Natural polysaccharide-based nanodrug delivery systems for treatment of diabetes. Polymers 2022, 14, 3217. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Z.; Thu, H.E.; Shuid, A.N.; Katas, H.; Hussain, F. Recent advances in polymer-based wound dressings for the treatment of diabetic foot ulcer: An overview of state-of-the-art. Curr. Drug Targets 2018, 19, 527–550. [Google Scholar] [CrossRef]
- Avramia, I.; Amariei, S. Spent brewer’s Yeast as a source of insoluble β-glucans. Int. J. Mol. Sci. 2021, 22, 825. [Google Scholar] [CrossRef] [PubMed]
- Maheshwari, G.; Sowrirajan, S.; Joseph, B. Extraction and isolation of β-glucan from grain sources-a review. J. Food Sci. 2017, 82, 1535–1545. [Google Scholar] [CrossRef] [Green Version]
- Glass, G.E. Cosmeceuticals: The principles and practice of skin rejuvenation by nonprescription topical therapy. Aesthet. Surg. J. Open Forum 2020, 2, ojaa038. [Google Scholar] [CrossRef]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Vaithanomsat, P.; Boonlum, N.; Trakunjae, C.; Apiwatanapiwat, W.; Janchai, P.; Boondaeng, A.; Phalinphattharakit, K.; Nimitkeatkai, H.; Jarerat, A. Functionality of Yeast β-Glucan Recovered from Kluyveromyces marxianus by Alkaline and Enzymatic Processes. Polymers 2022, 14, 1582. [Google Scholar] [CrossRef] [PubMed]
- Kofuji, K.; Aoki, A.; Tsubaki, K.; Konishi, M.; Isobe, T.; Murata, Y. Antioxidant activity of β-glucan. ISRN Pharm. 2012, 2012, 125864. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, S.D.; Cordeiro, S.L.; Cavalcanti, J.E.; Melchuna, K.M.; Lima, A.M.S.; Filho, I.A.; Medeiros, A.C.; Rocha, K.B.F.; Oliveira, E.M.; Faria, E.D.B.; et al. Effects of purified Saccharomyces cerevisiae (1→3)-β-glucan on venous ulcer healing. Int. J. Mol. Sci. 2012, 13, 8142–8158. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Meng, G.; Zhai, G.; Yang, Y.; Zhao, H.; Jia, L. Extraction, characterization and antioxidant activity of polysaccharides of spent mushroom compost of Ganoderma lucidum. Int. J. Biol. Macromol. 2016, 82, 432–439. [Google Scholar] [CrossRef]
- Kohguchi, M.; Kunikata, T.; Watanabe, H.; Kudo, N.; Shibuya, T.; Ishihara, T.; Iwaki, K.; Ikeda, M.; Fukuda, S.; Kurimoto, M. Immuno-potentiating effects of the antler-shaped fruiting body of Ganoderma lucidum (Rokkaku-Reishi). Biosci. Biotechnol. Biochem. 2004, 68, 881–887. [Google Scholar] [CrossRef] [Green Version]
- Cör, D.; Knez, Ž.; Hrnčič, M.K. Antitumour, antimicrobial, antioxidant and antiacetylcholinesterase effect of Ganoderma lucidum terpenoids and polysaccharides: A review. Molecules 2018, 23, 649. [Google Scholar] [CrossRef] [Green Version]
- Siwulski, M.; Sobieralski, K.; Golak-Siwulska, I.; Sokół, S.; Sękara, A. Ganoderma lucidum (Curt.: Fr.) Karst.—Health-promoting properties. A review. Herba. Pol. 2015, 61, 105–118. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Ma, W.; Wang, C.; Yang, X.; Lou, Y.; Xia, X.; Xu, H. Optimization of Enzyme-Assisted Extraction and Purification of Flavonoids from Pinus koraiensis Nut-Coated Film and Antioxidant Activity Evaluation. Molecules 2021, 26, 1950. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis of AOAC International, 17th ed.; AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Chaiyana, W.; Anuchapreeda, S.; Punyoyai, C.; Neimkhum, W.; Lee, K.-H.; Lin, W.-C.; Lue, S.-C.; Viernstein, H.; Mueller, M. Ocimum sanctum Linn. as a natural source of skin anti-ageing compounds. Ind. Crops Prod. 2019, 127, 217–224. [Google Scholar] [CrossRef]
- Kubo, I.; Kinst-Hori, I.; Chaudhuri, S.K.; Kubo, Y.; Sánchez, Y.; Ogura, T. Flavonols from heterotheca inuloides: Tyrosinase inhibitory activity and structural criteria. Bioorg Med Chem. 2000, 8, 1749–1755. [Google Scholar] [CrossRef]
- Saewan, N.; Koysomboon, S.; Chantrapromma, K. Anti-tyrosinase and anti-cancer activities of flavonoids from Blumea balsamifera DC. J. Med. Plant Res. 2011, 5, 1018–1025. [Google Scholar]
- Thring, T.S.A.; Hili, P.; Naughton, D.P. Anti-Collagenase, Anti-Elastase, and Anti-Oxidant Activities of Extracts from 21 Plants. BMC Complement Altern. Med. 2009, 9, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fotakis, G.; Timbrell, J.A. In vitro cytotoxicity assays: Comparison of LDH, neutral red, MTT and protein assay in hepatoma cell lines following exposure to cadmium chloride. Toxicol. Lett. 2006, 160, 171–177. [Google Scholar] [CrossRef]
- Somwongin, S.; Chantawannakul, P.; Chaiyana, W. Antioxidant activity and irritation property of venoms from Apis species. Toxicon 2018, 145, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Sangeethapriya, M.; Siddhuruju, P. Health related functional characteristics and antioxidant potential of mucilage (dietary fiber) from Zizyphus mauritiana fruits. Food Sci. Hum. Wellness 2014, 3, 79–88. [Google Scholar] [CrossRef] [Green Version]
- Daou, C.; Zhang, H. Physico-chemical properties and antioxidant activities of dietary fiber derived from defatted rice bran. Adv. J. Food Sci. Technol. 2011, 3, 339–347. [Google Scholar]
- Ogbe, A.O.; Obeka, A.D. Proximate, mineral and anti-nutrient composition of wild Ganoderma lucidum: Implication on its utilization in poultry production. Iran. J. Appl. Anim. Sci. 2013, 3, 161–166. [Google Scholar]
- Singh, R.; Kaur, N.; Shri, R.; Singh, A.P.; Dhingra, G.S. Proximate composition and element contents of selected species of Ganoderma with reference to dietary intakes. Environ. Monit. Assess. 2020, 192, 270. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. Mushroom polysaccharides: Chemistry and antiobesity, antidiabetes, anticancer, and antibiotic properties in cells, rodents, and humans. Foods 2016, 5, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, L.Z.; Lin, Z.B. Regulatory effect of Ganoderma lucidum polysaccharides on cytotoxic T-lymphocytes induced by dendritic cells in vitro. Acta Pharmacol. Sin. 2003, 24, 321–326. [Google Scholar]
- Liu, Y.; Tang, Q.; Zhang, J.; Xia, Y.; Yang, Y.; Wu, D.; Fan, H.; Cui, S.W. Triple helix conformation of β-d-glucan from Ganoderma lucidum and effect of molecular weight on its immunostimulatory activity. Int. J. Biol. Macromol. 2018, 15, 1064–1070. [Google Scholar] [CrossRef] [PubMed]
- Benito-Román, Ó.; Alonso, E.; Cocero, M.J.; Goto, M. β-Glucan recovery from Ganoderma lucidum by means of pressurized hot water and supercritical CO2. Food Bioprod. Process. 2016, 98, 21–28. [Google Scholar] [CrossRef]
- Hwang, I.W.; Kim, B.M.; Kim, Y.C.; Lee, S.H.; Chung, S.K. Improvement in β-glucan extraction from Ganoderma lucidum with high-pressure steaming and enzymatic pre-treatment. Appl. Biol. Chem. 2018, 61, 235–242. [Google Scholar] [CrossRef] [Green Version]
- Page, M.J.; Di Cera, E. Serine peptidases: Classification, structure, and function. Cell. Mol. Life Sci. 2008, 65, 1220–1236. [Google Scholar] [CrossRef] [PubMed]
- Veverka, M.; Dubaj, T.; Gallovič, J.; Jorík, V.; Veverková, E.; Mičušík, M.; Šimon, P. Beta-glucan complexes with selected nutraceuticals: Synthesis, characterization, and stability. J. Funct. Foods 2014, 8, 309–318. [Google Scholar] [CrossRef]
- Paulino, A.T.; Simionato, J.I.; Garcia, J.C.; Nozaki, J. Characterization of chitosan and chitin produced from silkworm crysalides. Carbohydr. Polym. 2006, 64, 98–103. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, C.; Liu, L.; Zhang, X.; Hou, Y.; Zhao, L. Characterization of chitin-glucan complex of Ganoderma lucidum extract and Its application as hemostatic hydrogel. Waste Biomass Valor. 2022, 13, 3297–3308. [Google Scholar] [CrossRef]
- Fairweather, J.K.; Him, J.L.K.; Heux, L.; Driguez, H.; Bulone, V. Structural characterization by 13C-NMR spectroscopy of products synthesized in vitro by polysaccharide synthases using 13C-enriched glycosyl donors: Application to a UDP-glucose:(1→3)-β-D-glucan synthase from blackberry (Rubus fruticosus). Glycobiology 2004, 14, 775–781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chuah, C.T.; Sarko, A. Triple-helical crystalline structure of curdlan and paramylon hydrates. Macromolecules 1983, 16, 1375–1382. [Google Scholar] [CrossRef]
- Van de Velde, K.; Kiekens, P. Structure analysis and degree of substitution of chitin, chitosan and dibutyrylchitin by FT-IR spectroscopy and solid state 13C NMR. Carbohydr. Polym. 2004, 58, 409–416. [Google Scholar] [CrossRef]
- Heux, L.; Brugnerotto, J.; Desbrières, J.; Versali, M.F.; Rinaudo, M. Solid state NMR for determination of degree of acetylation of chitin and chitosan. Biomacromolecules 2000, 1, 746–751. [Google Scholar] [CrossRef]
- Saito, H.; Ohki, T.; Sasaki, T. A 13C nuclear magnetic resonance study of polysaccharide gels: Molecular architecture in the gels consisting of fungal, branched (I-3)-B-D-glucans (lentinan and schizophyllan) as manifested by conformational changes induced by sodium hydroxide. Carbohydr. Res. 1979, 74, 227–240. [Google Scholar] [CrossRef]
- Kogan, G.; Alföldi, J.; Masler, L. Carbon-13 NMR spectroscopic investigation of two yeast cell wall b-D-glucans. Biopolymers 1988, 27, 1055–1063. [Google Scholar] [CrossRef]
- Seweryn, E.; Ziała, A.; Gamian, A. Health-Promoting of Polysaccharides Extracted from Ganoderma lucidum. Nutrients 2021, 13, 2725. [Google Scholar] [CrossRef] [PubMed]
- Binic, I.; Lazarevic, V.; Ljubenovic, M.; Mojsa, J.; Sokolovic, D. Skin ageing: Natural weapons and strategies. Evid. Based Complement. Alternat. Med. 2013, 2013, 827248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saeedi, M.; Eslamifar, M.; Khezri, K. Kojic acid applications in cosmetic and pharmaceutical preparations. Biomed. Pharmacother. 2019, 110, 582–593. [Google Scholar] [CrossRef] [PubMed]
- Chaiyana, W.; Charoensup, W.; Sriyab, S.; Punyoyai, C.; Neimkhum, W. Herbal extracts as potential antioxidant, anti-aging, anti-inflammatory, and whitening cosmeceutical ingredients. Chem. Biodivers. 2021, 18, e210024. [Google Scholar] [CrossRef]
- Madan, K.; Nanda, S. In-vitro evaluation of antioxidant, anti-elastase, anti-collagenase, anti-hyaluronidase activities of safranal and determination of its sun protection factor in skin photoaging. Bioorg. Chem. 2018, 77, 159–167. [Google Scholar] [CrossRef]
- Katsube, T.; Yamasaki, Y.; Iwamoto, M.; Oka, S. Hyaluronidase-inhibiting polysaccharide isolated and purified from hot water extract of sporophyll of Undaria pinnatifida. Food Sci. Technol. Res. 2003, 9, 25–29. [Google Scholar] [CrossRef] [Green Version]
- Sangthong, S.; Pintathong, P.; Pongsua, P.; Jirarat, A.; Chaiwut, P. Polysaccharides from Volvariella volvacea mushroom: Extraction, biological activities and cosmetic efficacy. J. Fungi 2022, 8, 572. [Google Scholar] [CrossRef]
- Du, B.; Bian, Z.; Xu, B. Skin health promotion effects of natural beta-glucan derived from cereals and microorganisms: A review. Phytother. Res. 2014, 28, 159–166. [Google Scholar] [CrossRef]
- Chen, J.; Lai, P.; Shen, H.; Zhen, H.; Fang, R. Effect of extraction methods on polysaccharide of Clitocybe maxima stipe. Adv. J. Food Sci. Technol. 2013, 5, 370–373. [Google Scholar] [CrossRef]



| BG Sources | BG (%w/w) | Carbohydrate (%w/w) | Fiber (%w/w) | Protein (%w/w) | Fat (%w/w) | Moisture (%w/w) | Ash (%w/w) |
|---|---|---|---|---|---|---|---|
| G. lucidum | 48.69 ± 0.63 a | 54.61 b | 30.63 ± 0.22 a | 7.47 ± 0.10 b | 0.12 ± 0.04 b | 6.79 ± 0.28 a | 0.38 ± 0.13 a |
| Commercial BG | 40.57 ± 0.90 b | 59.29 a | 20.76 ± 0.02 b | 11.61 ± 0.12 a | 2.61 ± 0.01 a | 5.30 ± 0.01 b | 0.43 ± 0.21 a |
| Antioxidant Activity | IC50 (mg/mL) | |
|---|---|---|
| G. lucidum BG | L-Ascorbic Acid | |
| DPPH• inhibition | 18.34 ± 5.77 * (R2 = 0.9584) | 0.05 ± 0.00 (R2 = 0.9584) |
| ABTS•+ inhibition | 0.07 ± 0.00 (R2 = 0.9947) | 0.06 ± 0.00 (R2 = 1.0000) |
| Ferric reducing power | 18.38 ± 1.68 * (R2 = 0.9584) | 0.03 ± 0.00 (R2 = 0.9985) |
| Anti-Tyrosinase Activity | Inhibition (%) | |
|---|---|---|
| G. lucidum BG | Kojic Acid | |
| Substrate: L-tyrosine Substrate: L-DOPA | 97.66 ± 0.59 * | 99.11 ± 0.48 |
| 24.13 ± 1.34 ** | 89.80 ± 0.17 | |
| Anti-Ageing Activity | Inhibition (%) | ||
|---|---|---|---|
| G. lucidum BG | EGCG | Oleanolic Acid | |
| Anti-collagenase | 21.03 ± 2.64 * | 66.07 ± 1.26 | ND |
| Anti-elastase | 26.19 ± 3.37 * | 89.61 ± 3.04 | ND |
| Anti-hyaluronidase | 29.26 ± 4.48 * | ND | 81.35 ± 1.55 |
| Samples | Before (0 min) | 5 min | 60 min | Irritation Score | |
|---|---|---|---|---|---|
| Negative control | 0.9% w/v Sodium chloride |  |  |  | 0.00 |
| Positive control | 1% w/v Sodium lauryl sulfate |  |  |  | 15.07 ± 0.08 |
| Sample | G. lucidum BG |  |  |  | 0.00 |
| Vehicle control | DI water |  |  |  | 0.00 |
| BG Sources | WHC (g/g) | WBC (g/g) | SC (mL/g) | OHC (g/g) |
|---|---|---|---|---|
| G. lucidum | 1.96 ± 0.01 a | 0.17 ± 0.01 a | 41.34 ± 0.53 a | 8.15 ± 0.04 a |
| Commercial BG | 1.97 ± 0.01 a | 0.13 ± 0.01 b | 41.79 ± 0.27 a | 3.00 ± 0.32 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vaithanomsat, P.; Boonlum, N.; Chaiyana, W.; Tima, S.; Anuchapreeda, S.; Trakunjae, C.; Apiwatanapiwat, W.; Janchai, P.; Boondaeng, A.; Nimitkeatkai, H.; et al. Mushroom β-Glucan Recovered from Antler-Type Fruiting Body of Ganoderma lucidum by Enzymatic Process and Its Potential Biological Activities for Cosmeceutical Applications. Polymers 2022, 14, 4202. https://doi.org/10.3390/polym14194202
Vaithanomsat P, Boonlum N, Chaiyana W, Tima S, Anuchapreeda S, Trakunjae C, Apiwatanapiwat W, Janchai P, Boondaeng A, Nimitkeatkai H, et al. Mushroom β-Glucan Recovered from Antler-Type Fruiting Body of Ganoderma lucidum by Enzymatic Process and Its Potential Biological Activities for Cosmeceutical Applications. Polymers. 2022; 14(19):4202. https://doi.org/10.3390/polym14194202
Chicago/Turabian StyleVaithanomsat, Pilanee, Nutthamon Boonlum, Wantida Chaiyana, Singkome Tima, Songyot Anuchapreeda, Chanaporn Trakunjae, Waraporn Apiwatanapiwat, Phornphimon Janchai, Antika Boondaeng, Hataitip Nimitkeatkai, and et al. 2022. "Mushroom β-Glucan Recovered from Antler-Type Fruiting Body of Ganoderma lucidum by Enzymatic Process and Its Potential Biological Activities for Cosmeceutical Applications" Polymers 14, no. 19: 4202. https://doi.org/10.3390/polym14194202
APA StyleVaithanomsat, P., Boonlum, N., Chaiyana, W., Tima, S., Anuchapreeda, S., Trakunjae, C., Apiwatanapiwat, W., Janchai, P., Boondaeng, A., Nimitkeatkai, H., & Jarerat, A. (2022). Mushroom β-Glucan Recovered from Antler-Type Fruiting Body of Ganoderma lucidum by Enzymatic Process and Its Potential Biological Activities for Cosmeceutical Applications. Polymers, 14(19), 4202. https://doi.org/10.3390/polym14194202