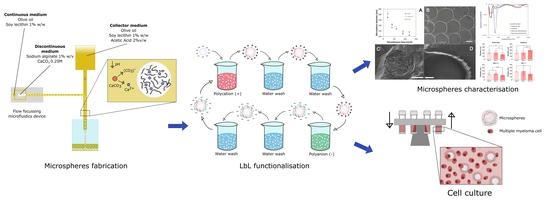
Stability of Biomimetically Functionalised Alginate Microspheres as 3D Support in Cell Cultures
Abstract
:1. Introduction
2. Hypotheses
3. Materials and Methods
3.1. Materials
3.2. Production of Alginate Microspheres
3.3. Layer by Layer Functionalisation
3.4. Microsphere Characterisation
3.5. Equilibrium Swelling
3.6. In Vitro Assay
3.7. Statistics
4. Results
4.1. Alginate Microsphere Production
4.2. Polyelectrolyte Multilayer Coatings
4.3. Coating Characteristics
4.4. Equilibrium Swelling
4.5. Cell Culture in the Microgel
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| CF (mL/h) | DF (mL/h) | Mean | SD | Std Error |
|---|---|---|---|---|
| 20 | 1 | 273.1 | 22.9 | 3.2 |
| 40 | 1 | 206.2 | 22.5 | 3.2 |
| 60 | 1 | 169.4 | 29.8 | 4.0 |
| 90 | 1 | 124.5 | 32.9 | 4.2 |
| 120 | 1 | 111.1 | 16.1 | 2.1 |
| 20 | 1.55 | 323.5 | 27.7 | 4.1 |
| 40 | 1.55 | 218.4 | 18.2 | 2.5 |
| 60 | 1.55 | 188.2 | 16.4 | 2.3 |
| 90 | 1.55 | 153.2 | 16.7 | 2.3 |
| 120 | 1.55 | 111.2 | 34.5 | 4.6 |
| 20 | 2 | 305.9 | 113.4 | 14.3 |
| 40 | 2 | 182.1 | 102.4 | 13.2 |
| 90 | 2 | 142.2 | 56.3 | 7.7 |
| 120 | 2 | 141.4 | 46.9 | 6.0 |

| Liquid Medium | Mean Day 0 | Mean Day 1 | Mean Day 14 | Significant Difference |
|---|---|---|---|---|
| pH 4 | 156.5 ± 43.9 | 168.6 ± 32.7 | 150.5 ± 39.3 | No |
| pH 5.5 | 160.1 ± 57.9 | 163.1 ± 36.6 | 180.2 ± 42.1 | No |
| pH 7 | 174.6 ± 40.0 | 177.8 ± 54.9 | 170.3 ± 42.0 | No |
| pH 9 | 175.3 ± 60.3 | 181.0 ± 63.8 | 186.7 ± 60.2 | No |
| RPMI 1460 | 244.8 ± 67.0 | 220.2 ± 74.6 | 161.6 ± 56.1 | Yes |
| DMEM w Phenol Red | 232.9 ± 50.1 | 220.3 ± 69.0 | 299.8 ± 53.9 | Yes |
| DMEM w/o Phenol Red | 185.2 ± 52.5 | 185.3 ± 50.9 | 178.7 ± 49.5 | No |
| PBS +/+ | 210.6 ± 42.1 | 207.7 ± 34.0 | 154.7 ± 30.2 | Yes |
| PBS −/− | 271.3 ± 13.2 | 290.0 ± 127.3 | 198.1 ± 61.7 | Yes |
| Liquid Medium | Mean Day 0 | Mean Day 1 | Mean Day 14 | Significant Difference |
|---|---|---|---|---|
| pH 4 | 262.9 ± 74.0 | 272.0 ± 72.6 | 287.1 ± 77.1 | No |
| pH 5.5 | 274.5 ± 94.5 | 233.1 ± 64.5 | 250.0 ± 31.5 | No |
| pH 7 | 246.7 ± 22.6 | 236.6 ± 34.2 | 258.9 ± 40.8 | No |
| pH 9 | 293.9 ± 64.7 | 293.3 ± 66.4 | 292.9 ± 88.6 | No |
| RPMI 1460 | 264.3 ± 17.2 | 268.8 ± 38.6 | 290.4 ± 58.9 | No |
| DMEM w Phenol Red | 252.5 ± 75.2 | 251.0 ± 81.2 | 279.8 ± 51.7 | No |
| DMEM w/o Phenol Red | 282.3 ± 45.0 | 287.9 ± 78.4 | 280.8 ± 79.2 | No |
| PBS +/+ | 271.6 ± 57.1 | 279.0 ± 39.2 | 270.5 ± 55.8 | No |
| PBS −/− | 288.9 ± 52.6 | 289.1 ± 54.5 | 286.6 ± 80.7 | No |
| Liquid Medium | Mean Day 0 | Mean Day 1 | Mean Day 14 | Significant Difference |
|---|---|---|---|---|
| pH 4 | 196.6 ± 34.4 | 200.5 ± 36.4 | 196.2 ± 34.1 | No |
| pH 5.5 | 221.1 ± 52.0 | 217.2 ± 56.2 | 219.9 ± 48.7 | No |
| pH 7 | 228.4 ± 54.8 | 240.2 ± 54.9 | 236.3 ± 54.1 | No |
| pH 9 | 221.0 ± 41.7 | 218.2 ± 52.3 | 209.6 ± 42.8 | No |
| RPMI 1460 | 187.6 ± 38.2 | 199.9 ± 32.6 | 199.0 ± 44.3 | No |
| DMEM w Phenol Red | 221.9 ± 41.6 | 197.5 ± 45.7 | 212.3 ± 51.5 | No |
| DMEM w/o Phenol Red | 215.0 ± 42.5 | 205.2 ± 41.7 | 209.0 ± 40.1 | No |
| PBS +/+ | 204.7 ± 50.9 | 217.3 ± 52.5 | 203.9 ± 44.6 | No |
| PBS −/− | 190.4 ± 34.0 | 191.0 ± 44.0 | 204.0 ± 40.7 | No |
References
- Hernández-González, A.C.; Téllez-Jurado, L.; Rodríguez-Lorenzo, L.M. Alginate Hydrogels for Bone Tissue Engineering, from Injectables to Bioprinting: A Review. Carbohydr. Polym. 2020, 229, 115514. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, P.; Kandasubramanian, B. Review of Alginate-Based Hydrogel Bioprinting for Application in Tissue Engineering. Biofabrication 2019, 11, 42001. [Google Scholar] [CrossRef] [PubMed]
- Abourehab, M.A.S.; Rajendran, R.R.; Singh, A.; Pramanik, S.; Shrivastav, P.; Ansari, M.J.; Manne, R.; Amaral, L.S.; Deepak, A. Alginate as a Promising Biopolymer in Drug Delivery and Wound Healing: A Review of the State-of-the-Art. Int. J. Mol. Sci. 2022, 23, 9035. [Google Scholar] [CrossRef] [PubMed]
- Andersen, T.; Auk-Emblem, P.; Dornish, M. 3D Cell Culture in Alginate Hydrogels. Microarrays 2015, 4, 133–161. [Google Scholar] [CrossRef]
- Cavo, M.; Caria, M.; Pulsoni, I.; Beltrame, F.; Fato, M.; Scaglione, S. A New Cell-Laden 3D Alginate-Matrigel Hydrogel Resembles Human Breast Cancer Cell Malignant Morphology, Spread and Invasion Capability Observed “in Vivo”. Sci. Rep. 2018, 8, 5333. [Google Scholar] [CrossRef] [Green Version]
- Jovic, T.H.; Kungwengwe, G.; Mills, A.C.; Whitaker, I.S. Plant-Derived Biomaterials: A Review of 3D Bioprinting and Biomedical Applications. Front. Mech. Eng. 2019, 5, 19. [Google Scholar] [CrossRef] [Green Version]
- Karmakar, S.; Manna, S.; Kabiraj, S.; Jana, S. Recent Progress in Alginate-Based Carriers for Ocular Targeting of Therapeutics. Food Hydrocoll. Health 2022, 2, 100071. [Google Scholar] [CrossRef]
- Genes, N.G.; Rowley, J.A.; Mooney, D.J.; Bonassar, L.J. Effect of Substrate Mechanics on Chondrocyte Adhesion to Modified Alginate Surfaces. Arch. Biochem. Biophys. 2004, 422, 161–167. [Google Scholar] [CrossRef]
- Mhanna, R.; Kashyap, A.; Palazzolo, G.; Vallmajo-Martin, Q.; Becher, J.; Möller, S.; Schnabelrauch, M.; Zenobi-Wong, M. Chondrocyte Culture in Three Dimensional Alginate Sulfate Hydrogels Promotes Proliferation While Maintaining Expression of Chondrogenic Markers. Tissue Eng. Part A 2014, 20, 1454–1464. [Google Scholar] [CrossRef] [Green Version]
- Pina, S.; Oliveira, J.M.; Reis, R.L. Natural-Based Nanocomposites for Bone Tissue Engineering and Regenerative Medicine: A Review. Adv. Mater. 2015, 27, 1143–1169. [Google Scholar] [CrossRef]
- Qazi, T.H.; Burdick, J.A. Granular Hydrogels for Endogenous Tissue Repair. Biomater. Biosyst. 2021, 1, 100008. [Google Scholar] [CrossRef]
- Caldwell, A.S.; Aguado, B.A.; Anseth, K.S. Designing Microgels for Cell Culture and Controlled Assembly of Tissue Microenvironments. Adv. Funct. Mater. 2020, 30, 1907670. [Google Scholar] [CrossRef]
- Marín-Payá, J.C.; Díaz-Benito, B.; Martins, L.A.; Clara-Trujillo, S.; Cordón, L.; Lanceros-Méndez, S.; Gallego Ferrer, G.; Sempere, A.; Gómez Ribelles, J.L. Biomimetic 3D Environment Based on Microgels as a Model for the Generation of Drug Resistance in Multiple Myeloma. Materials 2021, 14, 7121. [Google Scholar] [CrossRef]
- Clara-Trujillo, S.; Tolosa, L.; Cordón, L.; Sempere, A.; Gallego Ferrer, G.; Gómez Ribelles, J.L. Novel Microgel Culture System as Semi-Solid Three-Dimensional In vitro Model for the Study of Multiple Myeloma Proliferation and Drug Resistance. Biomater. Adv. 2022, 135, 212749. [Google Scholar] [CrossRef]
- Sherstneva, A.A.; Demina, T.S.; Monteiro, A.P.F.; Akopova, T.A.; Grandfils, C.; Ilangala, A.B. Biodegradable Microparticles for Regenerative Medicine: A State of the Art and Trends to Clinical Application. Polymers 2022, 14, 1314. [Google Scholar] [CrossRef]
- Clara-Trujillo, S.; Marín-Payá, J.C.; Cordón, L.; Sempere, A.; Gallego Ferrer, G.; Gómez Ribelles, J.L. Biomimetic Microspheres for 3D Mesenchymal Stem Cell Culture and Characterization. Colloids Surf. B Biointerfaces 2019, 177, 68–76. [Google Scholar] [CrossRef]
- Wawrzyńska, E.; Kubies, D. Alginate Matrices for Protein Delivery—A Short Review. Physiol. Res. 2018, 67, s319–s334. [Google Scholar] [CrossRef]
- García-Cruz, D.M.; Sardinha, V.; Escobar Ivirico, J.L.; Mano, J.F.; Gómez Ribelles, J.L. Gelatin Microparticles Aggregates as Three-Dimensional Scaffolding System in Cartilage Engineering. J. Mater. Sci. Mater. Med. 2013, 24, 503–513. [Google Scholar] [CrossRef] [Green Version]
- Zurriaga Carda, J.; Lastra, M.L.; Antolinos-Turpin, C.M.; Morales-Román, R.M.; Sancho-Tello, M.; Perea-Ruiz, S.; Milián, L.; Fernández, J.M.; Cortizo, A.M.; Carda, C.; et al. A Cell-Free Approach with a Supporting Biomaterial in the Form of Dispersed Microspheres Induces Hyaline Cartilage Formation in a Rabbit Knee Model. J. Biomed. Mater Res. B Appl. Biomater. 2020, 108, 1428–1438. [Google Scholar] [CrossRef]
- Shoichet, M.S.; Li, R.H.; White, M.L.; Winn, S.R. Stability of Hydrogels Used in Cell Encapsulation: An in Vitro Comparison of Alginate and Agarose. Biotechnol. Bioeng. 1996, 50, 374–381. [Google Scholar] [CrossRef]
- Augst, A.D.; Kong, H.J.; Mooney, D.J. Alginate Hydrogels as Biomaterials. Macromol. Biosci. 2006, 6, 623–633. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and Biomedical Applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [Green Version]
- Silva, J.M.; Reis, R.L.; Mano, J.F. Biomimetic Extracellular Environment Based on Natural Origin Polyelectrolyte Multilayers. Small 2016, 12, 4308–4342. [Google Scholar] [CrossRef]
- Cole, D.R.; Waterfall, M.; McIntyre, M.; Baird, J.C. Transplantation of Microcapsules (a Potential Bio-Artificial Organ): Biocompatibility and Host Reaction. J. Mater. Sci. Mater. Med. 1993, 4, 437–442. [Google Scholar] [CrossRef]
- Costa, R.R.; Castro, E.; Arias, F.J.; Rodríguez-Cabello, J.C.; Mano, J.F. Multifunctional Compartmentalized Capsules with a Hierarchical Organization from the Nano to the Macro Scales. Biomacromolecules 2013, 14, 2403–2410. [Google Scholar] [CrossRef]
- Yu, L.; Sun, Q.; Hui, Y.; Seth, A.; Petrovsky, N.; Zhao, C.X. Microfluidic Formation of Core-Shell Alginate Microparticles for Protein Encapsulation and Controlled Release. J. Colloid Interface Sci. 2019, 539, 497–503. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Wang, X.; Li, D.; Qin, J.; Zhang, M.; Wang, K.; Zhao, J.; Zhang, L. LBL Deposition of Chitosan/Heparin Bilayers for Improving Biological Ability and Reducing Infection of Nanofibers. Int. J. Biol. Macromol. 2020, 154, 999–1006. [Google Scholar] [CrossRef]
- Li, X.; Tang, J.; Bao, L.; Chen, L.; Hong, F.F. Performance Improvements of the BNC Tubes from Unique Double-Silicone-Tube Bioreactors by Introducing Chitosan and Heparin for Application as Small-Diameter Artificial Blood Vessels. Carbohydr. Polym. 2017, 178, 394–405. [Google Scholar] [CrossRef]
- Correia, C.R.; Reis, R.L.; Mano, J.F. Multilayered Hierarchical Capsules Providing Cell Adhesion Sites. Biomacromolecules 2013, 14, 743–751. [Google Scholar] [CrossRef] [Green Version]
- Ren, Y.; Xie, H.; Liu, X.; Bao, J.; Yu, W.; Ma, X. Comparative Investigation of the Binding Characteristics of Poly-L-Lysine and Chitosan on Alginate Hydrogel. Int. J. Biol. Macromol. 2016, 84, 135–141. [Google Scholar] [CrossRef]
- Algieri, C.; Donato, L.; Giorno, L. Tyrosinase Immobilized on a Hydrophobic Membrane. Biotechnol. Appl. Biochem. 2017, 64, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wu, F.; Ju, X.J.; Xie, R.; Wang, W.; Niu, C.H.; Chu, L.Y. Preparation of Monodisperse Calcium Alginate Microcapsules via Internal Gelation in Microfluidic-Generated Double Emulsions. J. Colloid Interface Sci. 2013, 404, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Anna, S.L.; Bontoux, N.; Stone, H.A. Formation of Dispersions Using “Flow Focusing” in Microchannels. Appl. Phys. Lett. 2003, 82, 364–366. [Google Scholar] [CrossRef]
- Fajardo, A.R.; Silva, M.B.; Lopes, L.C.; Piai, J.F.; Rubira, A.F.; Muniz, E.C. Hydrogel Based on an Alginate-Ca2+/Chondroitin Sulfate Matrix as a Potential Colon-Specific Drug Delivery System. RSC Adv. 2012, 2, 11095–11103. [Google Scholar] [CrossRef]
- Salaheldin, H.I.; Negm, A.; Osman, G.E.H. Porcine Skin Gelatin–Silver Nanocomposites: Synthesis, Characterisation, Cell Cytotoxicity, and Antibacterial Properties. IET Nanobiotechnol. 2017, 11, 957–964. [Google Scholar] [CrossRef] [PubMed]
- Sutirman, Z.A.; Sanagi, M.M.; Abd Karim, K.J.; Wan Ibrahim, W.A. Preparation of Methacrylamide-Functionalized Crosslinked Chitosan by Free Radical Polymerization for the Removal of Lead Ions. Carbohydr. Polym. 2016, 151, 1091–1099. [Google Scholar] [CrossRef] [PubMed]
- Tipson, R.S. Infrared Spectroscopy of Carbohydrates; National Institute of Standards and Technology (NIST): Gaithersburg, MD, USA, 1968. [Google Scholar]
- Liu, Y.; Wang, J.S.; Zhu, P.; Zhao, J.C.; Zhang, C.J.; Guo, Y.; Cui, L. Thermal Degradation Properties of Biobased Iron Alginate Film. J. Anal. Appl. Pyrolysis. 2016, 119, 87–96. [Google Scholar] [CrossRef] [Green Version]
- Dos Santos Araújo, P.; Belini, G.B.; Mambrini, G.P.; Yamaji, F.M.; Waldman, W.R. Thermal Degradation of Calcium and Sodium Alginate: A Greener Synthesis towards Calcium Oxide Micro/Nanoparticles. Int. J. Biol. Macromol. 2019, 140, 749–760. [Google Scholar] [CrossRef]
- Pathak, T.S.; Yun, J.H.; Lee, J.; Paeng, K.J. Effect of Calcium Ion (Cross-Linker) Concentration on Porosity, Surface Morphology and Thermal Behavior of Calcium Alginates Prepared from Algae (Undaria pinnatifida). Carbohydr. Polym. 2010, 81, 633–639. [Google Scholar] [CrossRef]
- Tian, G.; Ji, Q.; Xu, D.; Tan, L.; Quan, F.; Xia, Y. The Effect of Zinc Ion Content on Flame Retardance and Thermal Degradation of Alginate Fibers. Fibers Polym. 2013, 14, 767–771. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, J.C.; Zhang, C.J.; Guo, Y.; Cui, L.; Zhu, P.; Wang, D.Y. Bio-Based Nickel Alginate and Copper Alginate Films with Excellent Flame Retardancy: Preparation, Flammability and Thermal Degradation Behavior. RSC Adv. 2015, 5, 64125–64137. [Google Scholar] [CrossRef]
- Gåserød, O.; Smidsrød, O.; Skjåk-Bræk, G. Microcapsules of Alginate-Chitosan—I. A Quantitative Study of the Interaction between Alginate and Chitosan. Biomaterials 1998, 19, 1815–1825. [Google Scholar] [CrossRef]
- Erman, B.; Mark, J.E. Structures and Properties of Rubberlike Networks; Oxford University Press: Oxford, UK, 1997; ISBN 9780195082371. [Google Scholar]
- Flory, P.J.; Volkenstein, M. Statistical Mechanics of Chain Molecules; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1969; Volume 8. [Google Scholar]
- Clara-Trujillo, S.; Gallego Ferrer, G.; Gómez Ribelles, J.L. In Vitro Modeling of Non-Solid Tumors: How Far Can Tissue Engineering Go? Int. J. Mol. Sci. 2020, 21, 5747. [Google Scholar] [CrossRef]
- Ng, K.W.; Leong, D.T.W.; Hutmacher, D.W. The Challenge to Measure Cell Proliferation in Two and Three Dimensions. Tissue Eng. 2005, 11, 182–191. [Google Scholar] [CrossRef]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Briega, M.I.; Ródenas-Rochina, J.; Martins, L.A.; Lanceros-Méndez, S.; Gallego Ferrer, G.; Sempere, A.; Gómez Ribelles, J.L. Stability of Biomimetically Functionalised Alginate Microspheres as 3D Support in Cell Cultures. Polymers 2022, 14, 4282. https://doi.org/10.3390/polym14204282
García-Briega MI, Ródenas-Rochina J, Martins LA, Lanceros-Méndez S, Gallego Ferrer G, Sempere A, Gómez Ribelles JL. Stability of Biomimetically Functionalised Alginate Microspheres as 3D Support in Cell Cultures. Polymers. 2022; 14(20):4282. https://doi.org/10.3390/polym14204282
Chicago/Turabian StyleGarcía-Briega, María Inmaculada, Joaquín Ródenas-Rochina, Luis Amaro Martins, Senentxu Lanceros-Méndez, Gloria Gallego Ferrer, Amparo Sempere, and José Luís Gómez Ribelles. 2022. "Stability of Biomimetically Functionalised Alginate Microspheres as 3D Support in Cell Cultures" Polymers 14, no. 20: 4282. https://doi.org/10.3390/polym14204282
APA StyleGarcía-Briega, M. I., Ródenas-Rochina, J., Martins, L. A., Lanceros-Méndez, S., Gallego Ferrer, G., Sempere, A., & Gómez Ribelles, J. L. (2022). Stability of Biomimetically Functionalised Alginate Microspheres as 3D Support in Cell Cultures. Polymers, 14(20), 4282. https://doi.org/10.3390/polym14204282