Synergy in Aqueous Systems Containing Bioactive Ingredients of Natural Origin: Saponin/Pectin Mixtures
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Adsorption Layer Properties
3.1.1. Surface Tension
3.1.2. Surface Dilational Rheology
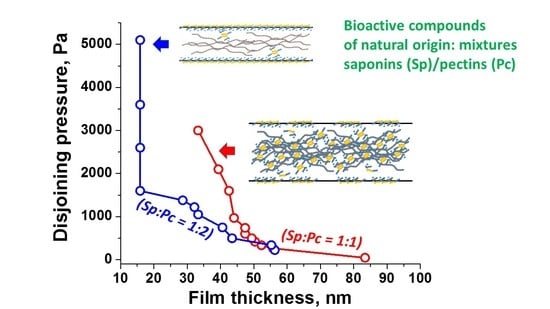
3.2. Microscopic Foam Films
4. Discussion
- Adsorption layer properties of aqueous solutions containing mixed formulations of Quillaja saponins and Apple pectins give plenty of indications for the onset of synergistic interactions in the investigated systems. The synergy is enhanced upon the increase of the pectin quantity at a fixed quantity of saponin (e.g., C(Sp) = 0.1 g/L, Figure 2). However, maximum effects are achieved at specific pectin concentrations. For example, surface dilational elasticity at the air/solution interface of the mixture is almost doubled at C(Pc) = 0.4, 0.5 and 1.0 g/L, as compared to the intrinsically high saponin-only solutions (Figure 4a,b), while the surface dilational viscosity is substantially decreased for all pectin quantities and there is no substantial dependence on the frequencies of the bubble’s deformation (Figure 5). Another interesting peculiarity is that the elasticity values at the highest frequencies are grouped in two tendencies: the maximum values are related to pectin additions of C(Pc) = 0.4, 0.5 and 1.0 g/L, while the next step (lower values) is registered at C(Pc) = 0.1, 0.3, 0.7 g/L of Apple pectin additions to the saponin aqueous solutions.
- The results on the drainage behavior and the stability of microscopic foam films support the notion of complex synergistic interactions in the mixed solutions. Thus, at a given saponin concentration, the foam films are significantly stabilized by the addition of various Apple pectin quantities. There exist optimum ratios of the components in the aqueous solution formulations, at which maximum synergy effects are observed: at C(Sp) = 0.1 g/L and C(Pc) = 0.2 g/L, the highest stability of the foam films is registered (Figure 8).
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Üstündağ, Ö.G.; Mazza, G. Saponins: Properties, Applications and Processing. Crit. Rev Food Sci. Nutr. 2007, 47, 231–258. [Google Scholar] [CrossRef] [PubMed]
- Böttcher, S.; Drusch, S. Saponins—Self-assembly and behavior at aqueous interfaces. Adv. Colloid Interface Sci. 2017, 243, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Vincken, J.-P.; Heng, L.; de Groot, A.; Gruppen, H. Saponins, classification and occurrence in the plant kingdom. Phytochemistry 2007, 68, 275–297. [Google Scholar] [CrossRef] [PubMed]
- Oleszek, W.A. Chromatographic determination of plant saponins. J. Chromatogr. A 2002, 967, 147–162. [Google Scholar] [CrossRef]
- Lacaille-Dubois, M.A.; Wagner, H. A review of the biological and pharmacological activities of saponins. Phytomedicine 1996, 2, 363–386. [Google Scholar] [CrossRef]
- Fleck, J.D.; Betti, A.H.; da Silva, F.P.; Troian, E.A.; Olivaro, C.; Ferreira, F.; Verza, S.G. Saponins from Quillaja saponaria and Quillaja brasiliensis: Particular Chemical Characteristics and Biological Activities. Molecules 2019, 24, 171. [Google Scholar] [CrossRef] [Green Version]
- Xu, M.; Wan, Z.; Yang, X. Recent Advances and Applications of Plant-Based Bioactive Saponins in Colloidal Multiphase Food Systems. Molecules 2021, 26, 6075. [Google Scholar] [CrossRef]
- Mohnen, D. Pectin structure and biosynthesis. Curr. Opin. Plant Biol. 2008, 11, 266–277. [Google Scholar] [CrossRef]
- Harholt, J.; Suttangkakul, A.; Scheller, H.V. Biosynthesis of Pectin. Plant Physiol. 2010, 153, 384–395. [Google Scholar] [CrossRef] [Green Version]
- Lutz, R.; Aserin, A.; Wicker, L.; Garti, N. Structure and physical properties of pectins with block-wise distribution of carboxylic acid groups. Food Hydrocoll. 2009, 23, 786–794. [Google Scholar] [CrossRef]
- Cui, J.; Zhao, C.; Feng, L.; Han, Y.; Du, H.; Xiao, H.; Zheng, J. Pectins from fruits: Relationships between extraction methods, structural characteristics, and functional properties. Trends Food Sci. Technol. 2021, 110, 39–54. [Google Scholar] [CrossRef]
- Sayah, M.Y.; Chabir, R.; Benyahia, H.; Kandri, Y.R.; Chahdi, F.O.; Touzani, H.; Errachidi, F. Yield, Esterification Degree and Mo-lecular Weight Evaluation of Pectins Isolated from Orange and Grapefruit Peels under Different Conditions. PLoS ONE 2016, 11, e0161751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Chen, Q.; Lü, X. Pectin extracted from apple pomace and citrus peel by subcritical water. Food Hydrocoll. 2014, 38, 129–137. [Google Scholar] [CrossRef]
- Kjøniksen, A.-L.; Hiorth, M.; Nyström, B. Temperature-induced association and gelation of aqueous solutions of pectin. A dynamic light scattering study. Eur. Polym. J. 2004, 40, 2427–2435. [Google Scholar] [CrossRef]
- Chan, S.Y.; Choo, W.S.; Young, D.J.; Loh, X.J. Pectin as a rheology modifier: Origin, structure, commercial production and rheology. Carbohydr. Polym. 2017, 161, 118–139. [Google Scholar] [CrossRef] [PubMed]
- Willats, W.G.T.; Knox, J.P.; Mikkelsen, J.D. Pectin: New insights into an old polymer are starting to gel. Trends Food Sci. Technol. 2006, 17, 97–104. [Google Scholar] [CrossRef]
- Echeverria, C.; Fernandes, S.N.; Godinho, M.H.; Borges, J.P.; Soares, P.I.P. Functional Stimuli-Responsive Gels: Hydrogels and Microgels. Gels 2018, 4, 54. [Google Scholar] [CrossRef] [Green Version]
- Hwang, J.; Kokini, L.J. Contribution to the side branches to rheological properties of pectin. Carbohydr. Polym. 1992, 19, 41–50. [Google Scholar] [CrossRef]
- Gawkowska, D.; Cybulska, J.; Zdunek, A. Structure-Related Gelling of Pectins and Linking with Other Natural Compounds: A Review. Polymers 2018, 10, 762. [Google Scholar] [CrossRef] [Green Version]
- Wusigale; Liang, L.; Luo, Y. Casein and pectin: Structures, interactions, and applications. Trends Food Sci. Technol. 2020, 97, 391–403. [Google Scholar] [CrossRef]
- Perez, A.A.; Sánchez, C.C.; Patino, J.M.R.; Rubiolo, A.C.; Santiago, L.G. Surface adsorption behaviour of milk whey protein and pectin mixtures under conditions of air–water interface saturation. Colloids Surf. B Biointerfaces 2011, 85, 306–315. [Google Scholar] [CrossRef]
- Liu, J.; Tan, J.; Hua, X.; Jiang, Z.; Wang, M.; Yang, R.; Cao, Y. Interfacial properties of ultrahigh methoxylated pectin. Int. J. Biol. Macromol. 2020, 152, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Santini, E.; Jarek, E.; Ravera, F.; Liggieri, L.; Warszynski, P.; Krzan, M. Surface properties and foamability of saponin and saponin-chitosan systems. Colloids Surf. B Biointerfaces 2019, 181, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Loglio, G.; Pandolfini, P.; Miller, R.; Makievski, A.V.; Ravera, F.; Ferrari, M.; Liggieri, L. Drop and bubble shape analysis as tool for dilational rheology studies of interfacial layers. In Novel Methods to Study Interfacial Layers, of Studies in Interface Science; Möbius, D., Miller, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2001; Volume 11, pp. 439–483. [Google Scholar]
- Liggieri, L.; Miller, R. Relaxation of surfactants adsorption layers at liquid interfaces. Curr. Opin. Colloid Interface Sci. 2010, 15, 256–263. [Google Scholar] [CrossRef]
- Liggieri, L.; Mileva, E.; Miller, R. The Surface Layer as the Basis for Foam Formation and Stability. In Foam Films and Foams; Exerowa, D., Gochev, G., Platikanov, D., Liggieri, L., Miller, R., Eds.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2019; Chapter 1; pp. 3–55. [Google Scholar]
- Scheludko, A.; Exerowa, D. Instrument for interferometric measuring of the thickness of microscopic foam layers. Commun. Inst. Chem. Bulg. Acad. Sci. 1959, 7, 123–131. [Google Scholar]
- Scheludko, A. Thin liquid films. Adv. Colloid Interface Sci. 1967, 1, 392–464. [Google Scholar] [CrossRef]
- Exerowa, D.; Kruglyakov, P.M. Experimental methods involved in the study of foam films. In Foams and Foam Films; Elsevier: Amsterdam, The Netherlands, 1998; Chapter 2; pp. 42–87. [Google Scholar]
- Platikanov, D.; Exerowa, D. Thin liquid films. Chapter 6. In Fundamentals of Interface and Colloid Science, 1st ed.; Lyklema, J., Ed.; Academic Press: Amsterdam, The Netherlands, 2005; Volume 5, pp. 6.1–6.91. [Google Scholar]
- Khristov, K.; Gotchew, G.; Petkova, H.; Levecke, B.; Tadros, T.; Exerowa, D. Foams stabilized by hydrophobically-modified inulin polymeric surfactant. Comptes Rendus De L’acad’Emie Bulg. Des Sci. 2013, 66, 3. [Google Scholar] [CrossRef]
- Platikanov, D.; Exerowa, D. Fundamentals of Foam Films. In Foam Films and Foams; Exerowa, D., Gochev, G., Platikanov, D., Liggieri, L., Miller, R., Eds.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2019; Chapter 3; pp. 77–98. [Google Scholar]
- Kolarov, T.; Cohen, R.; Exerowa, D. Direct measurement of disjoining pressure in black foam films. II. Films from nonionic surfactants. Colloids Surf. 1989, 42, 49–57. [Google Scholar] [CrossRef]
- Exerowa, D.; Kolarov, T.; Khristov, K. Direct measurement of disjoining pressure in black foam films. I. films from an ionic surfactant. Colloids Surf. 1987, 22, 171–185. [Google Scholar] [CrossRef]
- Khristov, K.; Exerowa, D.; Minkov, G. Critical capillary pressure for destruction of single foam films and foam: Effect of foam film size. Colloids Surf. A Physicochem. Eng. Asp. 2002, 210, 159–166. [Google Scholar] [CrossRef]
- Stanimirova, R.; Marinova, K.; Tcholakova, S.; Denkov, N.; Stoyanov, S.; Pelan, E. Surface rheology of saponin adsorption layers. Langmuir 2011, 27, 12486–12498. [Google Scholar] [CrossRef] [PubMed]
- Böttcher, S.; Drusch, S. Interfacial properties of saponin extracts and their impact on foam characteristics. Food Biophys. 2016, 11, 91–100. [Google Scholar] [CrossRef]
- Basak, S.; Annapure, U.S. Trends in “green” and novel methods of pectin modification—A review. Carbohydr. Polym. 2022, 278, 118967. [Google Scholar] [CrossRef] [PubMed]
- Zdunek, A.; Pieczywek, P.; Cybulska, J. The primary, secondary, and structures of higher levels of pectin polysaccharides. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1101–1117. [Google Scholar] [CrossRef]
- Thakur, B.R.; Singh, R.K.; Handa, A.K. Chemistry and uses of pectin—A review. Crit. Rev. Food Sci. Nutr. 1997, 37, 47–73. [Google Scholar] [CrossRef]










| Quillaja Saponins, C(Sp), g/L | Apple Pectins, C(Pc), g/L | Film Thickness h, nm |
|---|---|---|
| 0.025 | - | 98.7 ± 4.4 |
| 0.025 | 0.025 | 92.8 ± 3.4 |
| 0.1 | - | 98.7 ± 4.6 |
| - | 0.1 | 102.4 |
| 0.1 | 0.1 | 83.4 ± 1.9 |
| 0.1 | 0.2 | >102.4 |
| Quillaja Saponins C(Sp), g/L | Apple Pectins C(Pc), g/L | Critical Pressure of Film Rupture Pcr,film, Pa |
|---|---|---|
| 0.025 | - | 330 |
| 0.025 | 0.025 | 600–1000 |
| 0.1 | - | 220 |
| - | 0.1 | 400–1000 |
| 0.1 | 0.1 | 2000–3000 |
| 0.1 | 0.2 | 1000–5000 |
| 0.1 | 0.3 | 2000–3600 |
| 0.1 | 0.5 | 500–2500 |
| 0.1 | 0.7 | 2700–3700 |
| 0.1 | 1.0 | 500–3000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petkova, H.; Jarek, E.; Doychinov, M.; Krzan, M.; Mileva, E. Synergy in Aqueous Systems Containing Bioactive Ingredients of Natural Origin: Saponin/Pectin Mixtures. Polymers 2022, 14, 4362. https://doi.org/10.3390/polym14204362
Petkova H, Jarek E, Doychinov M, Krzan M, Mileva E. Synergy in Aqueous Systems Containing Bioactive Ingredients of Natural Origin: Saponin/Pectin Mixtures. Polymers. 2022; 14(20):4362. https://doi.org/10.3390/polym14204362
Chicago/Turabian StylePetkova, Hristina, Ewelina Jarek, Mitko Doychinov, Marcel Krzan, and Elena Mileva. 2022. "Synergy in Aqueous Systems Containing Bioactive Ingredients of Natural Origin: Saponin/Pectin Mixtures" Polymers 14, no. 20: 4362. https://doi.org/10.3390/polym14204362
APA StylePetkova, H., Jarek, E., Doychinov, M., Krzan, M., & Mileva, E. (2022). Synergy in Aqueous Systems Containing Bioactive Ingredients of Natural Origin: Saponin/Pectin Mixtures. Polymers, 14(20), 4362. https://doi.org/10.3390/polym14204362