Polylactic Acid Chemical Foaming Assisted by Solid-State Processing: Solid-State Shear Pulverization and Cryogenic Milling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Pre-Foaming Processing Methods
2.3. Compression Molding Foaming Process
2.4. Foam Analysis Methods
3. Results and Discussion
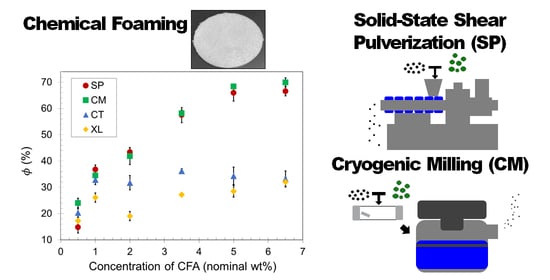
3.1. Density Reduction
3.2. Foam Morphology
3.3. Differential Scanning Calorimetry
3.4. Dynamic Mechanical Analysis Results
3.5. Static Compression Testing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Doyle, L.; Weidlich, I. Mechanical Behaviour of Polylactic Acid Foam as Insulation under Increasing Temperature. Environ. Clim. Technol. 2019, 23, 202–210. [Google Scholar] [CrossRef] [Green Version]
- Nofar, M.; Park, C.B. Poly (Lactic Acid) Foaming. Prog. Polym. Sci. 2014, 39, 1721–1741. [Google Scholar] [CrossRef]
- Lee, C.H.; Lee, K.-J.; Jeong, H.G.; Kim, S.W. Growth of Gas Bubbles in the Foam Extrusion Process. Adv. Polym. Technol. 2000, 19, 97–112. [Google Scholar] [CrossRef]
- Altan, M. Thermoplastic Foams: Processing, Manufacturing, and Characterization. In Recent Research in Polymerization; Cankaya, N., Ed.; InTech: London, UK, 2018; pp. 117–137. [Google Scholar]
- Chamas, A.; Moon, H.; Zheng, J.; Qiu, Y.; Tabassum, T.; Jang, J.H.; Abu-Omar, M.; Scott, S.L.; Suh, S. Degradation Rates of Plastics in the Environment. ACS Sustain. Chem. Eng. 2020, 8, 3494–3511. [Google Scholar] [CrossRef] [Green Version]
- Song, J.H.; Murphy, R.J.; Narayan, R.; Davies, G.B.H. Biodegradable and Compostable Alternatives to Conventional Plastics. Phil. Trans. R. Soc. B 2009, 364, 2127–2139. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Romain, C.; Williams, C.K. Sustainable Polymers from Renewable Resources. Nature 2016, 540, 354–362. [Google Scholar] [CrossRef]
- Zhao, S.; Malfait, W.J.; Guerrero-Alburquerque, N.; Koebel, M.M.; Nyström, G. Biopolymer Aerogels and Foams: Chemistry, Properties, and Applications. Angew. Chem. Int. Ed. 2018, 57, 7580–7608. [Google Scholar] [CrossRef]
- Gama, N.V.; Soares, B.; Freire, C.S.R.; Silva, R.; Neto, C.P.; Barros-Timmons, A.; Ferreira, A. Bio-Based Polyurethane Foams toward Applications beyond Thermal Insulation. Mater. Des. 2015, 76, 77–85. [Google Scholar] [CrossRef]
- Dorgan, J.R.; Lehermeier, H.; Mang, M. Thermal and Rheological Properties of Commercial-Grade Poly(Lactic Acid)s. J. Polym. Environ. 2000, 8, 1–9. [Google Scholar] [CrossRef]
- Cheng, Y.; Deng, S.; Chen, P.; Ruan, R. Polylactic Acid (PLA) Synthesis and Modifications: A Review. Front. Chem. China 2009, 4, 259–264. [Google Scholar] [CrossRef]
- Carothers, W.H.; Dorough, G.L.; van Natta, F.J. Studies of Polymerization and Ring Formation. X. The Reversible Polymerization of Six-Membered Cyclic Esters. J. Am. Chem. Soc. 1932, 54, 761–772. [Google Scholar] [CrossRef]
- ASTM D6691; Test Method for Determining Aerobic Biodegradation of Plastic Materials in the Marine Environment by a Defined Microbial Consortium or Natural Sea Water Inoculum. ASTM International: Conshohocken, PA, USA, 2009.
- Greene, J. PLA and PHA Biodegradation in the Marine Environment; California Department of Resources Recycling and Recovery: Sacramento, CA, U.S.A, 2012. [Google Scholar]
- Larsen, Å.; Neldin, C. Physical Extruder Foaming of Poly(Lactic Acid)-Processing and Foam Properties. Polym. Eng. Sci. 2013, 53, 941–949. [Google Scholar] [CrossRef]
- Rokkonen, T.; Peltola, H.; Sandquist, D. Foamability and Viscosity Behavior of Extrusion Foamed PLA–Pulp Fiber Biocomposites. J. Appl. Polym. Sci. 2019, 136, 48202. [Google Scholar] [CrossRef]
- Lee, S.-T.; Ramesh, N.S. (Eds.) Polymeric Foams: Mechanisms and Materials; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Kmetty, Á.; Litauszki, K.; Réti, D. Characterization of Different Chemical Blowing Agents and Their Applicability to Produce Poly(Lactic Acid) Foams by Extrusion. Appl. Sci. 2018, 8, 1960. [Google Scholar] [CrossRef] [Green Version]
- Yuan, H.; Liu, Z.; Ren, J. Preparation, Characterization, and Foaming Behavior of Poly(Lactic Acid)/Poly(Butylene Adipate- Co -Butylene Terephthalate) Blend. Polym. Eng. Sci. 2009, 49, 1004–1012. [Google Scholar] [CrossRef]
- Seo, J.-H.; Han, J.; Lee, K.S.; Cha, S.W. Combined Effects of Chemical and Microcellular Foaming on Foaming Characteristics of PLA (Poly Lactic Acid) in Injection Molding Process. Polym.-Plast. Technol. Eng. 2012, 51, 455–460. [Google Scholar] [CrossRef]
- Matuana, L.M.; Faruk, O.; Diaz, C.A. Cell Morphology of Extrusion Foamed Poly(Lactic Acid) Using Endothermic Chemical Foaming Agent. Bioresour. Technol. 2009, 100, 5947–5954. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhu, W.; Zhang, H.; Park, C.B. Continuous Processing of Low-Density, Microcellular Poly(Lactic Acid) Foams with Controlled Cell Morphology and Crystallinity. Chem. Eng. Sci. 2012, 75, 390–399. [Google Scholar] [CrossRef]
- Zhao, M.; Ding, X.; Mi, J.; Zhou, H.; Wang, X. Role of High-Density Polyethylene in the Crystallization Behaviors, Rheological Property, and Supercritical CO2 Foaming of Poly (Lactic Acid). Polym. Degrad. Stab. 2017, 146, 277–286. [Google Scholar] [CrossRef]
- Campuzano, J.F.; Lopez, I.D. Study of the Effect of Dicumyl Peroxide on Morphological and Physical Properties of Foam Injection Molded Poly(Lactic Acid)/Poly(Butylene Succinate) Blends. Express Polym. Lett. 2020, 14, 673–684. [Google Scholar] [CrossRef]
- Blumer, E.M.; Lynch, B.B.; Fielding, A.S.; Wakabayashi, K. Crystallinity and Property Enhancements in Neat Polylactic Acid by Chilled Extrusion: Solid-state Shear Pulverization and Solid-state/ Melt Extrusion. Polym. Eng. Sci. 2019, 59, E286–E295. [Google Scholar] [CrossRef]
- Lynch, B.B. The Crystallization Kinetics of Polylactic Acid (PLA) Processed Through Solid-State/Melt Extrusion. B.S. Thesis, Bucknell University, Lewisburg, PA, USA, 2014. [Google Scholar]
- Brunner, P. Overcoming Sustainability and Energy Challenges in Polymer Science Via Solid-State Shear Pulverization. Ph.D. Thesis, Northwestern University, Evanston, IL, USA, 2013. [Google Scholar]
- Ganglani, M.; Torkelson, J.M.; Carr, S.H.; Khait, K. Trace Levels of Mechanochemical Effects in Pulverized Polyolefins. J. Appl. Polym. Sci. 2001, 80, 671–679. [Google Scholar] [CrossRef]
- Diop, M.F.; Torkelson, J.M. Novel Synthesis of Branched Polypropylene via Solid-State Shear Pulverization. Polymer 2015, 60, 77–87. [Google Scholar] [CrossRef]
- Lebovitz, A.H.; Khait, K.; Torkelson, J.M. In Situ Block Copolymer Formation during Solid-State Shear Pulverization: An Explanation for Blend Compatibilization via Interpolymer Radical Reactions. Macromolecules 2002, 35, 9716–9722. [Google Scholar] [CrossRef]
- Tao, Y.; Lebovitz, A.H.; Torkelson, J.M. Compatibilizing Effects of Block Copolymer Mixed with Immiscible Polymer Blends by Solid-State Shear Pulverization: Stabilizing the Dispersed Phase to Static Coarsening. Polymer 2005, 46, 4753–4761. [Google Scholar] [CrossRef]
- Diop, M.F.; Burghardt, W.R.; Torkelson, J.M. Well-Mixed Blends of HDPE and Ultrahigh Molecular Weight Polyethylene with Major Improvements in Impact Strength Achieved via Solid-State Shear Pulverization. Polymer 2014, 55, 4948–4958. [Google Scholar] [CrossRef]
- Tao, Y.; Kim, J.; Torkelson, J.M. Achievement of Quasi-Nanostructured Polymer Blends by Solid-State Shear Pulverization and Compatibilization by Gradient Copolymer Addition. Polymer 2006, 47, 6773–6781. [Google Scholar] [CrossRef]
- Walker, A.M.; Tao, Y.; Torkelson, J.M. Polyethylene/Starch Blends with Enhanced Oxygen Barrier and Mechanical Properties: Effect of Granule Morphology Damage by Solid-State Shear Pulverization. Polymer 2007, 48, 1066–1074. [Google Scholar] [CrossRef]
- Iyer, K.A.; Lechanski, J.; Torkelson, J.M. Green Polypropylene/Waste Paper Composites with Superior Modulus and Crystallization Behavior: Optimizing Specific Energy in Solid-State Shear Pulverization for Filler Size Reduction and Dispersion. Compos.-A Appl. Sci. Manuf. 2016, 83, 47–55. [Google Scholar] [CrossRef]
- Wakabayashi, K.; Pierre, C.; Dikin, D.A.; Ruoff, R.S.; Ramanathan, T.; Brinson, L.C.; Torkelson, J.M. Polymer-Graphite Nanocomposites: Effective Dispersion and Major Property Enhancement via Solid-State Shear Pulverization. Macromolecules 2008, 41, 1905–1908. [Google Scholar] [CrossRef]
- Pujari, S.; Ramanathan, T.; Kasimatis, K.; Masuda, J.; Andrews, R.; Torkelson, J.M.; Brinson, L.C.; Burghardt, W.R. Preparation and Characterization of Multiwalled Carbon Nanotube Dispersions in Polypropylene: Melt Mixing versus Solid-State Shear Pulverization. J. Polym. Sci. B Polym. Phys. 2009, 47, 1426–1436. [Google Scholar] [CrossRef]
- Wakabayashi, K.; Brunner, P.J.; Masuda, J.; Hewlett, S.A.; Torkelson, J.M. Polypropylene-Graphite Nanocomposites Made by Solid-State Shear Pulverization: Effects of Significantly Exfoliated, Unmodified Graphite Content on Physical, Mechanical and Electrical Properties. Polymer 2010, 51, 5525–5531. [Google Scholar] [CrossRef]
- Iyer, K.A.; Torkelson, J.M. Novel, Synergistic Composites of Polypropylene and Rice Husk Ash: Sustainable Resource Hybrids Prepared by Solid-State Shear Pulverization. Polym. Compos. 2013, 34, 1211–1221. [Google Scholar] [CrossRef]
- Miu, E.V.; Fox, A.J.; Jubb, S.H.; Wakabayashi, K. Morphology and Toughness Enhancements in Recycled High-Density Polyethylene (rHDPE) via Solid-State Shear Pulverization (SSSP) and Solid-State/Melt Extrusion (SSME). J. Appl. Polym. Sci. 2016, 133, 43070. [Google Scholar] [CrossRef]
- Khait, K.; Torkelson, J.M. Solid-State Shear Pulverization of Plastics: A Green Recycling Process. Polym. -Plast. Technol. Eng. 1999, 38, 445–457. [Google Scholar] [CrossRef]
- Iyer, K.A.; Torkelson, J.M. Green Composites of Polypropylene and Eggshell: Effective Biofiller Size Reduction and Dispersion by Single-Step Processing with Solid-State Shear Pulverization. Compos. Sci. Technol. 2014, 102, 152–160. [Google Scholar] [CrossRef]
- Iyer, K.A.; Flores, A.M.; Torkelson, J.M. Comparison of Polyolefin Biocomposites Prepared with Waste Cardboard, Microcrystalline Cellulose, and Cellulose Nanocrystals via Solid-State Shear Pulverization. Polymer 2015, 75, 78–87. [Google Scholar] [CrossRef]
- Wakabayashi, K.; Vancoillie, S.H.E.; Assfaw, M.G.; Choi, D.H.; Desplentere, F.; Van Vuure, A.W. Low-temperature Compounding of Flax Fibers with Polyamide 6 via Solid-state Shear Pulverization: Towards Viable Natural Fiber Composites with Engineering Thermoplastics. Polym. Compos. 2019, 40, 3285–3295. [Google Scholar] [CrossRef]
- Brunner, P.J.; Torkelson, J.M. Microcrystalline Cellulose Composites of Poly(Lactic Acid)/Poly(Ethylene Glycol) or Polypropylene Created via Solid-State Shear Pulverization. ANTEC Proc. 2011, 1, 655–660. [Google Scholar]
- Henry, M.F. Solid-State Compatibilization of Immiscible Polymer Blends: Cryogenic Milling and Solid-State Shear Pulverization. M.S. Thesis, Bucknell University, Lewisburg, PA, USA, 2010. [Google Scholar]
- Hubert, P.J.; Kathiresan, K.; Wakabayashi, K. Filler Exfoliation and Dispersion in Polypropylene/as-Received Graphite Nanocomposites via Cryogenic Milling. Polym. Eng. Sci. 2011, 51, 2273–2281. [Google Scholar] [CrossRef]
- Gabriel, M.C.; Mendes, L.B.; de Melo Carvalho, B.; Pinheiro, L.A.; Capochi, J.D.T.; Kubaski, E.T.; Cintho, O.M. High-Energy Mechanical Milling of Ultra-High Molecular Weight Polyethylene (UHMWPE). Mater. Sci. Forum 2010, 660–661, 325–328. [Google Scholar] [CrossRef]
- Segura González, E.A.; Olmos, D.; González-Gaitano, G.; Orgaz, B.; González-Benito, J. Effect of Kaolin Nanofiller and Processing Conditions on the Structure, Morphology, and Biofilm Development of Polylactic Acid. J. Appl. Polym. Sci. 2015, 132, 42676. [Google Scholar] [CrossRef]
- Candau, N.; Oguz, O.; León Albiter, N.; Förster, G.; Maspoch, M.L. Poly (Lactic Acid)/Ground Tire Rubber Blends Using Peroxide Vulcanization. Polymers 2021, 13, 1496. [Google Scholar] [CrossRef]
- Ingeo Biopolymer 2003D Technical Data Sheet; NatureWorks LLC: Minnetonka, MN, USA, 2015.
- Bhatti, A.S.; Goddard, R.J.; O’Donnell, G. The Thermal Decomposition of Azodicarbonamide. Thermochim. Acta 1984, 76, 63–77. [Google Scholar] [CrossRef]
- Yang, S.; Wu, Z.-H.; Yang, W.; Yang, M.-B. Thermal and Mechanical Properties of Chemical Crosslinked Polylactide (PLA). Polym. Test. 2008, 27, 957–963. [Google Scholar] [CrossRef]
- Onffroy, P.R.; Miu, E.V.; Confer, W.J.; Darkes-Burkey, C.M.; Holler, W.C.; Wakabayashi, K. Residence Time Distribution and Specific Mechanical Energy in Solid-state Shear Pulverization: Processing-structure-property Relationships in a Chilled Extruder. Polym. Eng. Sci. 2020, 60, 503–511. [Google Scholar] [CrossRef]
- Katiyar, N.K.; Biswas, K.; Tiwary, C.S. Cryomilling as Environmentally Friendly Synthesis Route to Prepare Nanomaterials. Int. Mater. Rev. 2021, 66, 493–532. [Google Scholar] [CrossRef]
- Matuana, L.M.; Diaz, C.A. Study of Cell Nucleation in Microcellular Poly(Lactic Acid) Foamed with Supercritical CO2 through a Continuous-Extrusion Process. Ind. Eng. Chem. Res. 2010, 49, 2186–2193. [Google Scholar] [CrossRef]
- Reglero Ruiz, J.A.; Vincent, M.; Agassant, J.-F.; Sadik, T.; Pillon, C.; Carrot, C. Polymer Foaming with Chemical Blowing Agents: Experiment and Modeling. Polym. Eng. Sci. 2015, 55, 2018–2029. [Google Scholar] [CrossRef]
- Miltz, J.; Ramon, O. Characterization of Stress Relaxation Curves of Plastic Foams. Polym. Eng. Sci. 1986, 26, 1305–1309. [Google Scholar] [CrossRef]
- Khemani, K.C. (Ed.) Polymeric Foams: Science and Technology; American Chemical Society: Washington, DC, USA, 1997. [Google Scholar]
- Jang, L.K.; Fletcher, G.K.; Monroe, M.B.B.; Maitland, D.J. Biodegradable Shape Memory Polymer Foams with Appropriate Thermal Properties for Hemostatic Applications. J. Biomed. Mater. Res. 2020, 108, 1281–1294. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hikima, Y.; Ohshima, M.; Yusa, A.; Yamamoto, S.; Goto, H. Development of a Simplified Foam Injection Molding Technique and Its Application to the Production of High Void Fraction Polypropylene Foams. Ind. Eng. Chem. Res. 2017, 56, 13734–13742. [Google Scholar] [CrossRef]
- Zhai, W.; Ko, Y.; Zhu, W.; Wong, A.; Park, C.B. A Study of the Crystallization, Melting, and Foaming Behaviors of Polylactic Acid in Compressed CO2. Int. J. Mol. Sci. 2009, 10, 5381–5397. [Google Scholar] [CrossRef]
- Barzegari, M.R.; Rodrigue, D. The Effect of Injection Molding Conditions on the Morphology of Polymer Structural Foams. Polym. Eng. Sci. 2009, 49, 949–959. [Google Scholar] [CrossRef]
- Standau, T.; Zhao, C.; Murillo Castellón, S.; Bonten, C.; Altstädt, V. Chemical Modification and Foam Processing of Polylactide (PLA). Polymers 2019, 11, 306. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Zhang, X.; Wang, D. Tailoring Crystallization: Towards High-Performance Poly(Lactic Acid). Adv. Mater. 2014, 26, 6905–6911. [Google Scholar] [CrossRef]
- Tomin, M.; Kmetty, Á. Polymer Foams as Advanced Energy Absorbing Materials for Sports Applications—A Review. J. Appl. Polym. Sci. 2022, 139, 51714. [Google Scholar] [CrossRef]
- Yang, B.; Zuo, Y.; Chang, Z. Evaluation of Energy Absorption Capabilities of Polyethylene Foam under Impact Deformation. Materials 2021, 14, 3613. [Google Scholar] [CrossRef]
- Shaid Sujon, M.A.; Islam, A.; Nadimpalli, V.K. Damping and Sound Absorption Properties of Polymer Matrix Composites: A Review. Polym. Test. 2021, 104, 107388. [Google Scholar] [CrossRef]
- Bizhani, H.; Katbab, A.A.; Lopez-Hernandez, E.; Miranda, J.M.; Lopez-Manchado, M.A.; Verdejo, R. Preparation and Characterization of Highly Elastic Foams with Enhanced Electromagnetic Wave Absorption Based On Ethylene-Propylene-Diene-Monomer Rubber Filled with Barium Titanate/Multiwall Carbon Nanotube Hybrid. Polymers 2020, 12, 2278. [Google Scholar] [CrossRef]
- Zhang, L.; Jeon, H.K.; Malsam, J.; Herrington, R.; Macosko, C.W. Substituting Soybean Oil-Based Polyol into Polyurethane Flexible Foams. Polymer 2007, 48, 6656–6667. [Google Scholar] [CrossRef]
- Song, P.; Zhang, Y.; Luo, Y.; Liao, X.; Tang, W.; Yang, J.; Tian, C.; Li, G. Design of Lightweight Silicone Rubber Foam for Outstanding Deformation Recoverability Based on Supercritical CO2 Foaming Technology. J. Mater. Sci. 2022, 57, 2292–2304. [Google Scholar] [CrossRef]
- Miltz, J.; Ramon, O. Energy Absorption Characteristics of Polymeric Foams Used as Cushioning Materials. Polym. Eng. Sci. 1990, 30, 129–133. [Google Scholar] [CrossRef]








| Processing Type | Enthalpy Type (J/g) | Concentration of CFA (Nominal wt%) | |||||
|---|---|---|---|---|---|---|---|
| 0.5 wt% | 1.0 wt% | 2.0 wt% | 3.5 wt% | 5.0 wt% | 6.5 wt% | ||
| SSSP (SP) | ∆Hm | 15.5 | 16.1 | 17.2 | 15.7 | 17.1 | 14.0 |
| ∆Hcc | 10.0 | 9.0 | 9.6 | 8.3 | 9.2 | 8.0 | |
| Hmc | 5.5 | 7.1 | 7.6 | 7.4 | 7.9 | 6.0 | |
| Cryomill (CM) | ∆Hm | 14.8 | 16.6 | 16.0 | 14.2 | 17.2 | 15.0 |
| ∆Hcc | 6.7 | 8.8 | 7.5 | 7.8 | 12.0 | 8.6 | |
| Hmc | 8.1 | 7.8 | 8.5 | 6.4 | 5.2 | 6.4 | |
| Control (CT) | ∆Hm | 3.1 | 5.3 | 5.2 | 5.5 | 4.4 | 4.7 |
| ∆Hcc | 1.4 | 2.7 | 4.7 | 3.2 | 3.5 | 2.2 | |
| Hmc | 1.7 | 2.6 | 0.5 | 2.3 | 0.9 | 2.5 | |
| Crosslinked (XL) | ∆Hm | 17.4 | 19.0 | 16.5 | 20.5 | 16.4 | 17.7 |
| ∆Hcc | 2.1 | 4.7 | 1.3 | 2.5 | 2.0 | 0.7 | |
| Hmc | 15.3 | 14.3 | 15.2 | 18.0 | 14.4 | 17.0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onffroy, P.R.; Herrold, N.T.; Goehrig, H.G.; Yuen, K.; Wakabayashi, K. Polylactic Acid Chemical Foaming Assisted by Solid-State Processing: Solid-State Shear Pulverization and Cryogenic Milling. Polymers 2022, 14, 4480. https://doi.org/10.3390/polym14214480
Onffroy PR, Herrold NT, Goehrig HG, Yuen K, Wakabayashi K. Polylactic Acid Chemical Foaming Assisted by Solid-State Processing: Solid-State Shear Pulverization and Cryogenic Milling. Polymers. 2022; 14(21):4480. https://doi.org/10.3390/polym14214480
Chicago/Turabian StyleOnffroy, Philip R., Nathan T. Herrold, Harrison G. Goehrig, Kalie Yuen, and Katsuyuki Wakabayashi. 2022. "Polylactic Acid Chemical Foaming Assisted by Solid-State Processing: Solid-State Shear Pulverization and Cryogenic Milling" Polymers 14, no. 21: 4480. https://doi.org/10.3390/polym14214480
APA StyleOnffroy, P. R., Herrold, N. T., Goehrig, H. G., Yuen, K., & Wakabayashi, K. (2022). Polylactic Acid Chemical Foaming Assisted by Solid-State Processing: Solid-State Shear Pulverization and Cryogenic Milling. Polymers, 14(21), 4480. https://doi.org/10.3390/polym14214480