3.1. Box–Behnken Design
Box–Behnken design is a rotatable plan which means that the prediction variance depends only on the distance of the design point from the center of the design [
31]. The greater the absolute value of the coded variable, the greater the variance of the calculated value. The variance is calculated from the last three experiments nos. 13, 14, and 15 in which the independent variables are kept constant at level 0, so the differences in the results of these experiments are not variable. Hence, the difference in the results of these experiments allows the variance to be calculated.
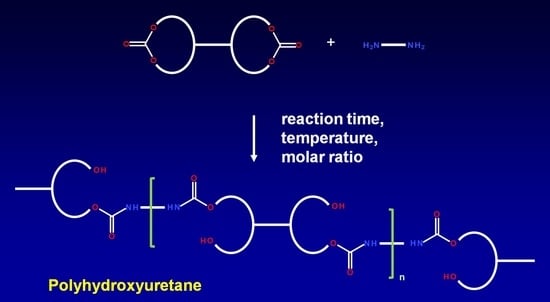
The low level for the reaction time was 60 min, because our initial experiments revealed that such time was sufficient for cyclic carbonates to converse completely, i.e., ~1800 cm
−1 band disappeared on the FTIR spectrum. The high level was 120 min as a longer reaction time may cause allophanates formation. The standard level (0) was 90 min which is an arithmetic mean of high- and low-level values (Equation (2)).
The low level for the reaction temperature was 120 °C due to the melting point of DCBPA [
30]. The high level was 160 °C to avoid favorable allophanate formation at higher temperatures which might reduce the properties of a PHU. The standard level (0) was an arithmetic mean of high and low levels (Equation (3)).
All experimental runs were performed at constant 1,6-hexamethylenediamine content 0.10 moles, while the variable was BGC/DCBPA carbonates molar ratio. The high level (+1) was coded for 0.10 moles BGC, low level (−1) for 0.10 moles DCBPA and the standard level (0) was coded for 0.05 moles BGC: 0.05 moles DCBPA mixture.
3.4. Thermal Properties
In order to determine the synthesis conditions on the thermal properties of the PHUs, the polymers were subjected to DSC analysis. Measurements were performed in the range −50 to 150 °C. Glass transition temperatures (
Tgs) are presented in
Table 5. A DSC curve recorded for the PHU synthesized in experiment no. 11 is shown in
Figure 7, while
Tgs of all the obtained PHUs are combined in
Figure 8. In the applied temperature range only glass transition occurred in all cases, so it proves that the PHUs are amorphous.
The data in
Table 5 and
Figure 8 indicate three types of the polymers resulting from the reagents’ compositions. The first group are thermoplastics synthesized in run nos. 5 (120 °C, 90 min), 6 (160 °C, 90 min), 9 (140 °C, 60 min), 10 (140 °C, 120 min), where DCBPA was used, which exhibit
Tgs in the range between 0 °C and 10 °C. What is worth noting is that both the temperature increases and prolonged reaction time resulted in just slight increase in the
Tg.
The experiment run nos. 1 (120 °C, 60 min), 2 (160 °C, 60 min), 3 (120 °C, 120 min), 4 (160 °C, 120 min), 13, 14 and 15 (140 °C, 90 min) with equimolar mixture of BGC/DCBPA yielded PHUs of Tgs between 40 °C and 50 °C. The effect of reaction temperature increase resulted in a slight decrease in the Tg of the product.
The highest Tgs (60–70 °C) exhibited the polymers obtained in the run nos. 7 (120 °C, 90 min), 8 (160 °C, 90 min), 11 (140 °C, 60 min) and 12 (140 °C, 120 min). Apparently, the significant factor affecting Tg was the temperature, while reaction time was insignificant.
As the lowest Tgs were obtained for purely aliphatic PHUs, the above results are coherent with the commonly agreed knowledge on the influence of polymer structure on its thermal properties.
3.6. Mathematical Relations between Variables
This work is focused on the analysis of how reaction time, temperature and reagents’ molar ratios affect the properties of products, i.e., PHUs. The Box–Behnken design provides a mathematical relation between the variables in the form of polynomial a quadratic equation (Equation (4)).
The polynomial equation for all the individual responses in terms of coded independent factors is as follows:
where:
is the computed response of the system,
is the coefficient of the equation,
b0 is the free term,
is the independent variable,
is the number of the independent variables, and
e is the constant.
The free term
b0 is computed from Equation (5) as the arithmetic mean of values of a given trait, while Equation (6) describes determination of the coefficients in monomials bearing one independent variable in power of one. Equation (7) is used in determination of the coefficients in monomials bearing one independent variable in power of two, and Equation (8) concerns determination of the coefficients in monomials with the product of two independent variables in power of one. The approach yields the interactions between variables.
where
is the arithmetic mean of
y results of the population when coded factors are equal 0;
is the experiment number (population);
is the constants as shown in
Table 6.
The Box–Behnken design allows computing the variations for the determined correlation coefficients. Equation (9) yields the variation for the free term in Equation (5), Equation (10) yields coefficients of the monomials in Equation (6), while Equations (11) and (12) yield the coefficients determined in Equations (7) and (8), respectively.
where:
is the variation determined for
y results of the population when the coded factors are equal 0,
is the number of experiments when the coded factors are equal 0.
Next, the computed correlation coefficients and their variations were the subject of the null hypothesis (
H0) test (Equation (13)) against the alternative hypothesis (
Ha) (Equation (14)). For each of the coefficients
was computed (Equation (15)) and compared to
(Equation (16)). If
, then the null hypothesis was rejected, while
bi ≠ 0 condition was accepted at a 95% confidence level. Hence, the variable bound to this coefficient affects a given trait. However, if
then the null hypothesis must not be rejected and
bi = 0. Then, it can be concluded at a 95% confidence level that the variable bound to this coefficient has no influence on a given trait.
The abovementioned approach was applied to determine the effect of the independent variables (reaction time, temperature and reagents’ molar ratio) on the properties of PHUs such as viscosity, glass transition temperature and mechanical performance.
The
tcrit was calculated using MS Excel T.INV function bounding probability (0.05) and degree of freedom (2), then
. The coefficients in polynomial describing
Tg’s and their significance are presented in
Table 7. The data indicate that
b0,
b3 and
b33 only are significant, hence, the carbonate used in synthesis had a significant effect on the
Tg of PHUs. The effect of the other variables, i.e., temperature (
b1,
b11) and time (
b2,
b22), was not significant. The null hypothesis was also tested for the monomials describing interactions of the variables (
b12,
b13,
b23). Equation (17) describes relations between significant monomials, and
Figure 9 shows the plot of the function in the range between −1 and +1.
The calculated coefficients of the polynomial describing the effect on PHUs mechanical properties are presented in
Table 8. It is clear that only
b0 and
b3 had significant effect, i.e., composition of carbonate reagents (
b3). The variables temperature (
b1,
b11) and time (
b2,
b22) were found to be insignificant. The null hypothesis was tested for the monomials describing interactions between the variables (
b12,
b13,
b23). Equation (18) defines relations between significant monomials, and
Figure 10 shows the plot of the function in the range between −1 and +1.
As the data in
Table 9 indicate, the only insignificant monomial was found for the interaction of time and temperature (
b12). For the other monomials the alternative hypothesis (
Ha) was accepted. The strongest effect on the viscosity was observed for the carbonate(s) used in the syntheses as the respective coefficients are significantly higher. Equation (19 defines relations between significant monomials, and
Figure 11 shows the plot of the function in the range between −1 and +1.
Each point was described by 4 variables. The independent variables were ascribed to X, Y and Z axes, while viscosity was illustrated by the color. It is clear that the aromatic DCBPA (coded variable +1) yielded the PHUs of viscosities nearly two orders of magnitude higher when compared to other experiment runs. Additionally, coded variables at levels 0 and −1 result in viscosities differencing by decimals of order of magnitude. In such cases, a quadratic polynomial has an insufficiently flexible function to accurately model the existing relations.
In order to verify the accuracy of the model, synthesis under the optimized conditions was performed. Namely, temperature 120 °C, reaction time 78 min, and carbonate mixture composition: 0.06 moles DCBPA, 0.40 moles BGC and 0.10 moles HMDA. The true properties of the resultant PHU are compared with the modeled ones in
Table 10.
As far as the shear strength and the
Tg are concerned, the values predicted by the model provided satisfactory coherence with the experiment. Lesser accordance was found for the viscosity. The gap might have been caused by a limited applicability of a quadratic polynomial to that specific interaction. One of the possible explanations is that the viscosity can be affected by moisture absorption [
40].