Comprehensive Enzymatic Conversion of Starch for the Food Industry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Particle Size of Native Starch
2.3. Mechanical Treatment
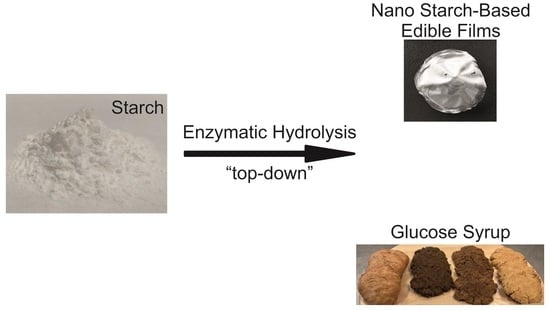
2.4. Producing Starch Nanoparticles and Glucose Syrup
2.5. The Zeta Potential and Particle Size of Starch Nanoparticles
2.6. Film Preparation
2.7. Moisture Content
2.8. Film Thickness
2.9. X-ray Diffraction Analysis
2.10. Scanning Electron Microscopy (SEM)
2.11. Differential Scanning Calorimetry (DSC)
2.12. Preparing Confectionery Products Using the Resulting Glucose Syrup
2.13. Quality Evaluation of the Confectionery Products
2.14. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Properties of Native Starch
3.2. Production of Nano-Sized Starch Particles and Glucose Syrup
3.3. Preparing Native and Nano Starch-Based Films from Tapioca Starch
3.4. Physicochemical Properties of Films Prepared from Native Tapioca Starch and Nano Starch
3.5. Producing Glucose Syrup for Confectionery Products from the Supernatant
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Gupta, V.; Biswas, D.; Roy, S. A Comprehensive review of biodegradable polymer-based films and coatings and their food packaging applications. Materials 2022, 15, 5899. [Google Scholar] [CrossRef] [PubMed]
- Guilbert, S.; Gontard, N. 16—Agro-polymers for edible and biodegradable films: Review of agricultural polymeric materials, physical and mechanical characteristics. In Food Science and Technology, 1st ed.; Han, J.H., Ed.; Academic Press: Cambridge, MA, USA, 2005; Volume 16, pp. 263–276. [Google Scholar] [CrossRef]
- Akopova, T.A.; Popyrina, T.N.; Demina, T.S. Mechanochemical transformations of polysaccharides: A systematic review. Int. J. Mol. Sci. 2022, 23, 10458. [Google Scholar] [CrossRef] [PubMed]
- Horstmann, S.W.; Lynch, K.M.; Arendt, E.K. Starch characteristics linked to gluten-free products. Foods 2017, 6, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhull, S.B.; Chandak, A.; Collins, M.N.; Bangar, S.P.; Chawla, P.; Singh, A. Lotus seed starch: A novel functional ingredient with promising properties and applications in food—A review. Starch 2022, 74, 2200064. [Google Scholar] [CrossRef]
- Mariadoss, A.V.A.; Saravanakumar, K.; Sathiyaseelan, A.; Venkatachalam, K.; Wang, M.-H. Folic acid functionalized starch encapsulated green synthesized copper oxide nanoparticles for targeted drug delivery in breast cancer therapy. Int. J. Biol. Macromol. 2020, 164, 2073–2084. [Google Scholar] [CrossRef] [PubMed]
- Clerici, M.T.P.S.; Schmiele, M. Starches for Food Application: Chemical, Technological and Health Properties, 1st ed.; Academic Press: London, UK, 2019; 460p. [Google Scholar]
- Gamage, A.; Liyanapathiranage, A.; Manamperi, A.; Gunathilake, C.; Mani, S.; Merah, O.; Madhujith, T. Applications of starch biopolymers for a sustainable modern agriculture. Sustainability 2022, 14, 6085. [Google Scholar] [CrossRef]
- Nowak, T.; Mazela, B.; Olejnik, K.; Peplińska, B.; Perdoch, W. Starch-silane structure and its influence on the hydrophobic properties of paper. Molecules 2022, 27, 3136. [Google Scholar] [CrossRef]
- Bhatt, P.; Kumar, V.; Goel, R.; Sharma, S.K.; Kaushik, S.; Sharma, S.; Shrivastava, A.; Tesema, M. Structural modifications and strategies for native starch for applications in advanced drug delivery. Biomed. Res. Int. 2022, 2022, 2188940. [Google Scholar] [CrossRef] [PubMed]
- Ojogbo, E.; Ogunsona, E.O.; Mekonnen, T.H. Chemical and physical modifications of starch for renewable polymeric materials. Mater. Today Sustain. 2020, 7–8, 100028. [Google Scholar] [CrossRef]
- Dome, K.; Podgorbunskikh, E.; Bychkov, A.; Lomovsky, O. Changes in the crystallinity degree of starch having different types of crystal structure after mechanical pretreatment. Polymers 2020, 12, 641. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Garcia, M.E.; Hernandez-Landaverde, M.A.; Delgado, J.M.; Ramirez-Gutierrez, C.F.; Ramirez-Cardona, M.; Millan-Malo, B.M.; Londoño-Restrepo, S.M. Crystalline structures of the main components of starch. Curr. Opin. Food Sci. 2021, 37, 107–111. [Google Scholar] [CrossRef]
- Huang, J.; Zhao, L.; Man, J.; Wang, J.; Zhou, W.; Huai, H.; Wei, C. Comparison of physicochemical properties of B-type nontraditional starches from different sources. Int. J. Biol. Macromol. 2015, 78, 165–172. [Google Scholar] [CrossRef]
- He, W.; Wei, C. Progress in C-type starches from different plant sources. Food Hydrocoll. 2017, 73, 162–175. [Google Scholar] [CrossRef]
- Robyt, J. Starch: Structure, properties, chemistry, and enzymology. In Glycoscience; Fraser-Reid, B.O., Tatsuta, K., Thiem, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 1437–1472. [Google Scholar] [CrossRef]
- Chaudhary, P.; Fatima, F.; Kumar, A. Relevance of nanomaterials in food packaging and its advanced future prospects. J. Inorg. Organomet. Polym. Mater. 2020, 30, 5180–5192. [Google Scholar] [CrossRef]
- do Val Siqueira, L.; Arias, C.I.L.F.; Maniglia, B.C.; Tadini, C.C. Starch-based biodegradable plastics: Methods of production, challenges and future perspectives. Curr. Opin. Food Sci. 2021, 38, 122–130. [Google Scholar] [CrossRef]
- Chavan, P.; Sinhmar, A.; Sharma, S.; Dufresne, A.; Thory, R.; Kaur, M.; Sandhu, K.S.; Nehra, M.; Nain, V. Nanocomposite starch films: A new approach for biodegradable packaging materials. Starch Stärke 2022, 74, 2100302. [Google Scholar] [CrossRef]
- Liu, D.; Zhao, P.; Chen, J.; Yan, Y.; Wu, Z. Recent advances and applications in starch for intelligent active food packaging: A review. Foods 2022, 11, 2879. [Google Scholar] [CrossRef] [PubMed]
- Colivet, J.; Carvalho, R.A. Hydrophilicity and physicochemical properties of chemically modified cassava starch films. Ind. Crops Prod. 2017, 95, 599–607. [Google Scholar] [CrossRef]
- Siroha, A.K.; Bangar, S.P.; Sandhu, K.S.; Trif, M.; Kumar, M.; Guleria, P. Effect of cross-linking modification on structural and film-forming characteristics of pearl millet (Pennisetum glaucum L.) starch. Coatings 2021, 11, 1163. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, Y.; Chen, G. A comparative study on the starch-based biocomposite films reinforced by nanocellulose prepared from different non-wood fibers. Cellulose 2019, 26, 2425–2435. [Google Scholar] [CrossRef]
- Condés, M.C.; Añón, M.C.; Dufresne, A.; Mauri, A.N. Composite and nanocomposite films based on amaranth biopolymers. Food Hydrocoll. 2018, 74, 159–167. [Google Scholar] [CrossRef]
- Lin, Q.; Ji, N.; Li, M.; Dai, L.; Xu, X.; Xiong, L.; Sun, Q. Fabrication of debranched starch nanoparticles via reverse emulsification for improvement of functional properties of corn starch films. Food Hydrocoll. 2020, 104, 105760. [Google Scholar] [CrossRef]
- Sharma, I.; Sinhmar, A.; Thory, R.; Sandhu, K.S.; Kaur, M.; Nain, V.; Pathera, A.K.; Chavan, P. Synthesis and characterization of nano starch-based composite films from kidney bean (Phaseolus vulgaris). J. Food Sci. Technol. 2021, 58, 2178–2185. [Google Scholar] [CrossRef] [PubMed]
- García, N.L.; Ribba, L.; Dufresne, A.; Aranguren, M.I.; Goyanes, S. Physico-mechanical properties of biodegradable starch nanocomposites. Macromol. Mater. Eng. 2009, 294, 169–177. [Google Scholar] [CrossRef]
- Oh, S.M.; Lee, B.H.; Seo, D.H.; Choi, H.W.; Kim, B.Y.; Baik, M.Y. Cite this article Starch nanoparticles prepared by enzymatic hydrolysis and self-assembly of short-chain glucans. Food Sci. Biotechnol. 2020, 29, 585–598. [Google Scholar] [CrossRef]
- Hassan, N.A.; Darwesh, O.M.; Smuda, S.S.; Altemimi, A.B.; Hu, A.; Cacciola, F.; Haoujar, I.; Abedelmaksoud, T.G. Recent trends in the preparation of nano-starch particles. Molecules 2022, 27, 5497. [Google Scholar] [CrossRef]
- Kim, H.-Y.; Lee, J.H.; Kim, J.-Y.; Lim, W.-J.; Lim, S.-T. Characterization of nanoparticles prepared by acid hydrolysis of various starches. Starch Stärke 2012, 64, 367–373. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Park, D.-J.; Lim, S.-T. Fragmentation of waxy rice starch granules by enzymatic hydrolysis. Cereal Chem. 2008, 85, 182–187. [Google Scholar] [CrossRef]
- Hao, Y.; Chen, Y.; Li, Q.; Gao, Q. Preparation of starch nanocrystals through enzymatic pretreatment from waxy potato starch. Carbohydr. Polym. 2018, 184, 171–177. [Google Scholar] [CrossRef]
- Dukare, A.S.; Arputharaj, A.; Bharimalla, A.K.; Saxena, S.; Vigneshwaran, N. Nanostarch production by enzymatic hydrolysis of cereal and tuber starches. Carbohydr. Polym. Technol. Appl. 2021, 2, 100121. [Google Scholar] [CrossRef]
- Paolucci-Jeanjean, D.; Belleville, M.P.; Rios, G.M. A comprehensive study of the loss of enzyme activity in a continuous membrane reactor—Application to starch hydrolysis. J. Chem. Technol. Biotechnol. 2001, 76, 273–278. [Google Scholar] [CrossRef]
- Eggleston, G.; Legendre, B.; Godshall, M.A. Sugar and other sweeteners. In Handbook of Industrial Chemistry and Biotechnology; Kent, J., Bommaraju, T., Barnicki, S., Eds.; Springer: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Bueno-Zabala, K.A.; Lopresto, C.G.; Calabro, V.; Curcio, S.; Ruiz-Colorado, A.A.; Chakraborty, S. Optimized production of glucose syrup and enzyme membrane reactor using in situ product recovery. Ind. Eng. Chem. Res. 2020, 59, 21305–21311. [Google Scholar] [CrossRef]
- Hull, P. Glucose syrups: Technology and Application, 1st ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2010; 392p. [Google Scholar]
- Paulino, B.N.; Molina, G.; Pastore, G.M.; Bicas, J.L. Current perspectives in the biotechnological production of sweetening syrups and polyols. Curr. Opin. Food Sci. 2021, 41, 36–43. [Google Scholar] [CrossRef]
- Kramer, H.; Steiner, A. Notes on the Hagedorn-Jensen method for the determination of blood-sugar. Biochem. J. 1931, 25, 161–165. [Google Scholar] [CrossRef] [Green Version]
- Podgorbunskikh, E.M.; Dome, K.V.; Bukhtoyarov, V.; Bychkov, A.L. X-ray diffraction for detecting starch adulteration and measuring the crystallinity indices of the polymorphic modifications of starch. Health Food Biotechnol. 2022, 4, 62–70. [Google Scholar] [CrossRef]
- Filipčev, B.; Bodroža-Solarov, M.; Šimurina, O.; Cvetković, B. Use of sugar beet molasses in processing of gingerbread type biscuits: Effect on quality characteristics, nutritional profile, and bioavailability of calcium and iron. Acta Aliment. 2012, 41, 494–505. [Google Scholar] [CrossRef]
- Wang, Y.; Lian, J.; Wan, J.; Ma, Y.; Zhang, Y. A supramolecular structure insight for conversion property of cellulose in hot compressed water: Polymorphs and hydrogen bonds changes. Carbohydr. Polym. 2015, 133, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Roy, K.; Thory, R.; Sinhmar, A.; Pathera, A.K.; Nain, V. Development and characterization of nano starch-based composite films from mung bean (Vigna radiata). Int. J. Biol. Macromol. 2020, 144, 242–251. [Google Scholar] [CrossRef]
- Wali, J.A.; Milner, A.J.; Luk, A.W.S.; Pulpitel, T.J.; Dodgson, T.; Facey, H.J.; Wahl, D.; Kebede, M.A.; Senior, A.M.; Sullivan, M.A.; et al. Impact of dietary carbohydrate type and protein–carbohydrate interaction on metabolic health. Nat. Metab. 2021, 3, 810–828. [Google Scholar] [CrossRef]
- Fakayode, O.A.; Peter, E.E.; Ojoawo, O.O. Rheological characterization and modelling of glucose syrup production process from selected agricultural crops. Agric. Eng. Int. CIGR J. 2019, 21, 127–134. [Google Scholar]
- Glendinning, J.I.; Williams, N. Consumption of glucose syrup enhances glucose tolerance in mice. Physiol. Behav. 2022, 256, 113954. [Google Scholar] [CrossRef] [PubMed]
- Frolova, Y.V. Russian market of fermented kombucha beverages. Vopr. Pitan. 2022, 91, 115–158. [Google Scholar] [CrossRef] [PubMed]
- Rosa-Sibakov, N.; Sorsamäki, L.; Immonen, M.; Nihtilä, H.; Maina, N.H.; Siika-Aho, M.; Katina, K.; Nordlund, E. Functionality and economic feasibility of enzymatically hydrolyzed waste bread as a sugar replacer in wheat bread making. J. Food Process. Preserv. 2022, 46, e16378. [Google Scholar] [CrossRef]





| Time, h | Yield Reducing Sugar, % | CrI, % | Average Particle Size, nm |
|---|---|---|---|
| 0 | - | 41 ± 1 | >1000 |
| 6 | 18 ± 1 | 52 ± 3 | 642 ± 40 |
| 12 | 40 ± 1 | 50 ± 2 | 428 ± 30 |
| 24 | 59 ± 2 | 49 ± 2 | 189 ± 17 |
| 96 | 64 ± 3 | 47 ± 2 | 66 ± 6 |
| Sample | Film Thickness, µm | Moisture Content, % | CrI, % | T1, °C | T2, °C | T3, °C | ΔH, J/g |
|---|---|---|---|---|---|---|---|
| Native starch film | 13.6 ± 1.4 | 31.8 ± 0.1 | 11.4 ± 0.4 | 290.3 | 296.3 | 300.8 | −18.54 |
| Amorphous starch film | 7.0 ± 0.7 | 20.3 ± 0.1 | 18.5 ± 0.9 | 289.4 | 294.7 | 304.7 | −53.60 |
| Nano starch-based films | 4.7 ± 0.7 | 14.9 ± 0.1 | 21.6 ± 0.7 | 289.8 | 292.7 | 297.6 | −19.70 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Podgorbunskikh, E.; Sapozhnikov, A.; Kuskov, T.; Gurova, D.; Kopylova, A.; Bychkov, A.; Lomovsky, O. Comprehensive Enzymatic Conversion of Starch for the Food Industry. Polymers 2022, 14, 4575. https://doi.org/10.3390/polym14214575
Podgorbunskikh E, Sapozhnikov A, Kuskov T, Gurova D, Kopylova A, Bychkov A, Lomovsky O. Comprehensive Enzymatic Conversion of Starch for the Food Industry. Polymers. 2022; 14(21):4575. https://doi.org/10.3390/polym14214575
Chicago/Turabian StylePodgorbunskikh, Ekaterina, Aleksandr Sapozhnikov, Timofei Kuskov, Daria Gurova, Anastasiia Kopylova, Aleksey Bychkov, and Oleg Lomovsky. 2022. "Comprehensive Enzymatic Conversion of Starch for the Food Industry" Polymers 14, no. 21: 4575. https://doi.org/10.3390/polym14214575
APA StylePodgorbunskikh, E., Sapozhnikov, A., Kuskov, T., Gurova, D., Kopylova, A., Bychkov, A., & Lomovsky, O. (2022). Comprehensive Enzymatic Conversion of Starch for the Food Industry. Polymers, 14(21), 4575. https://doi.org/10.3390/polym14214575