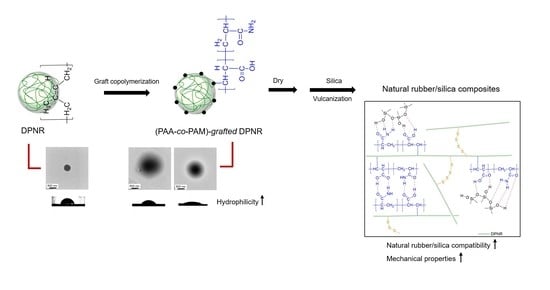
Preparation of Poly(acrylic acid-co-acrylamide)-Grafted Deproteinized Natural Rubber and Its Effect on the Properties of Natural Rubber/Silica Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Poly(acrylic acid-co-acrylamide)-Grafted Deproteinized Natural Rubber ((PAA-co-PAM)-DPNR)
2.3. Characterization of (PAA-co-PAM)-DPNR
2.3.1. Determination of Monomer Conversion
2.3.2. Determination of Grafting Efficiency and Grafting Percentage
2.3.3. Fourier-Transform Infrared Spectroscopy
2.3.4. Morphological Analysis
2.3.5. Particles Size Distribution
2.3.6. Zeta Potential
2.3.7. Contact Angle
2.3.8. Differential Scanning Calorimetry
2.3.9. Thermogravimetric Analysis
2.4. Preparation of Silica-Filled (PAA-co-PAM)-DPNR Composites
2.5. Characterization of the Silica-Filled (PAA-co-PAM)-DPNR Composites
2.5.1. Cure Characteristics
2.5.2. Swelling Ratio
2.5.3. Morphology
2.5.4. Mechanical Properties
2.5.5. Dynamic Mechanical Property
3. Results and Discussion
3.1. Modification of Deproteinized Natural Rubber by Graft Copolymerization with Comonomer of Acrylic and Acrylamide
3.1.1. Effect of Reaction Temperature
3.1.2. Effect of Initiator Content
3.1.3. Effect of Monomer Content
3.2. Characterization of (PAA-co-PAM)-Modified Deproteinized Natural Rubber
3.2.1. Chemical Structure of the (PAA-co-PAM)-Modified DPNR
3.2.2. Morphology of (PAA-co-PAM)-DPNR
3.2.3. Particle Size and Surface Properties
3.2.4. Thermal Properties
3.3. Preparation and Properties of the Silica-Filled (PAA-co-PAM)-DPNR Composites
3.3.1. Cure Characteristics
3.3.2. Morphology of Silica-Filled Natural Rubber Composites
3.3.3. Mechanical Properties of Composites
3.3.4. Dynamic Mechanical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Supramaniam, J.; Low, D.Y.S.; Wong, S.K.; Leo, B.F.; Goh, B.H.; Tang, S.Y. Nano-engineered ZnO/CNF-based epoxidized natural rubber with enhanced strength for novel Self-healing glove fabrication. Chem. Eng. J. 2022, 437, 135440. [Google Scholar] [CrossRef]
- Sreelekshmi, R.V.; Sudha, J.D.; Menon, A.R.R. Novel organomodified kaolin/silica hybrid fillers in natural rubber and its blend with polybutadiene rubber. Polym. Bull. 2017, 74, 783–801. [Google Scholar] [CrossRef]
- Chagas, P.A.M.; Schneider, R.; Santos, D.M.; Otuka, A.J.G.; Mendonça, C.R.; Correa, D.S. Bilayered electrospun membranes composed of poly(lactic-acid)/natural rubber: A strategy against curcumin photodegradation for wound dressing application. React. Funct. Polym. 2021, 163, 104889. [Google Scholar] [CrossRef]
- Tanan, W.; Panichpakdee, J.; Suwanakood, P.; Saengsuwan, S. Biodegradable hydrogels of cassava starch-g-polyacrylic acid/natural rubber/polyvinyl alcohol as environmentally friendly and highly efficient coating material for slow-release urea fertilizers. J. Ind. Eng. Chem. 2021, 101, 237–252. [Google Scholar] [CrossRef]
- Fang, Q.; Song, B.; Tee, T.-T.; Sin, L.T.; Hui, D.; Bee, S.-T. Investigation of dynamic characteristics of nano-size calcium carbonate added in natural rubber vulcanizate. Compos. Part B Eng. 2014, 60, 561–567. [Google Scholar] [CrossRef]
- Meng, Z.; Li, J.; Zou, Y.; Li, N.; Fu, X.; Zhang, R.; Hu, S.; Liu, Q. Advanced montmorillonite modification by using corrosive microorganisms as an alternative filler to reinforce natural rubber. Appl. Clay Sci. 2022, 225, 106534. [Google Scholar] [CrossRef]
- Keawkumay, C.; Jarukumjorn, K.; Wittayakun, J.; Suppakarn, N. Influences of surfactant content and type on physical properties of natural rubber/organoclay nanocomposites. J. Polym. Res. 2012, 19, 9917. [Google Scholar] [CrossRef]
- Zheng, J.; Han, D.; Zhao, S.; Ye, X.; Wang, Y.; Wu, Y.; Dong, D.; Liu, J.; Wu, X.; Zhang, L. Constructing a Multiple Covalent Interface and Isolating a Dispersed Structure in Silica/Rubber Nanocomposites with Excellent Dynamic Performance. ACS Appl. Mater. Interfaces 2018, 10, 19922–19931. [Google Scholar] [CrossRef]
- Rovani, S.; Santos, J.J.; Corio, P.; Fungaro, D.A. Highly Pure Silica Nanoparticles with High Adsorption Capacity Obtained from Sugarcane Waste Ash. ACS Omega 2018, 3, 2618–2627. [Google Scholar] [CrossRef]
- Shen, Y. Rice Husk Silica-Derived Nanomaterials for Battery Applications: A Literature Review. J. Agric. Food Chem. 2017, 65, 995–1004. [Google Scholar] [CrossRef]
- Tang, Y.P.; Chung, T.S.; Weber, M.; Maletzko, C. Development of Novel Diol-Functionalized Silica Particles toward Fast and Efficient Boron Removal. Ind. Eng. Chem. Res. 2017, 56, 11618–11627. [Google Scholar] [CrossRef]
- Go, M.R.; Bae, S.H.; Kim, H.J.; Yu, J.; Choi, S.J. Interactions between Food Additive Silica Nanoparticles and Food Matrices. Front. Microbiol. 2017, 8, 1013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thuong, N.T.; Dung, T.A.; Yusof, N.H.; Kawahara, S. Controlling the size of silica nanoparticles in filler nanomatrix structure of natural rubber. Polymer 2020, 195, 122444. [Google Scholar] [CrossRef]
- Poompradub, S.; Thirakulrati, M.; Prasassarakich, P. In situ generated silica in natural rubber latex via the sol–gel technique and properties of the silica rubber composites. Mater. Chem. Phys. 2014, 144, 122–131. [Google Scholar] [CrossRef]
- Kim, M.C.; Adhikari, J.; Kim, J.K.; Saha, P. Preparation of novel bio-elastomers with enhanced interaction with silica filler for low rolling resistance and improved wet grip. J. Clean. Prod. 2019, 208, 1622–1630. [Google Scholar] [CrossRef]
- Balachandrakurup, V.; Gopalakrishnan, J. Enhanced performance of cellulose nanofibre reinforced styrene butadiene rubber nanocomposites modified with epoxidised natural rubber. Ind. Crops Prod. 2022, 183, 114935. [Google Scholar] [CrossRef]
- Somseemee, O.; Sae-Oui, P.; Siriwong, C. Bio-based epoxidized natural rubber/chitosan/cellulose nanocrystal composites for enhancing mechanical properties, self-healing behavior and triboelectric nanogenerator performance. Cellulose 2022, 29, 8675–8693. [Google Scholar] [CrossRef]
- Wongthong, P.; Nakason, C.; Pan, Q.; Rempel, G.L.; Kiatkamjornwong, S. Modification of deproteinized natural rubber via grafting polymerization with maleic anhydride. Eur. Polym. J. 2013, 49, 4035–4046. [Google Scholar] [CrossRef]
- Jayadevan, J.; Rosamma, A.; Unnikrishnan, G. Deproteinised natural rubber latex grafted poly(dimethylaminoethyl methacrylate)-poly(vinyl alcohol) blend membranes: Synthesis, properties and application. Int. J. Biol. Macromol. 2017, 107, 1821–1834. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Xiang, Y.; Xu, Y.; Wei, J.; Zhang, Z.; Li, L.; Li, J. Poly-acrylic acid grafted natural rubber for multi-coated slow release compound fertilizer: Preparation, properties and slow-release characteristics. Int. J. Biol. Macromol. 2020, 146, 540–548. [Google Scholar] [CrossRef]
- Wilson, B.K.; Prud’homme, R.K. Processing Chitosan for Preparing Chitosan-Functionalized Nanoparticles by Polyelectrolyte Adsorption. Langmuir 2021, 37, 8517–8524. [Google Scholar] [CrossRef] [PubMed]
- Konko, I.; Guriyanova, S.; Boyko, V.; Sun, L.; Liu, D.; Reck, B.; Men, Y. Role of the Hydrophilic Latex Particle Surface in Water Diffusion into Films from Waterborne Polymer Colloids. Langmuir 2019, 35, 6075–6088. [Google Scholar] [CrossRef]
- Sennakesavan, G.; Mostakhdemin, M.; Dkhar, L.K.; Seyfoddin, A.; Fatihhi, S.J. Acrylic acid/acrylamide based hydrogels and its properties-A review. Polym. Degrad. Stab. 2020, 180, 109308. [Google Scholar] [CrossRef]
- Viriyakitpattana, N.; Sunintaboon, P. Synthesis of crosslinked poly(methacrylic acid) shell/lipid core colloidal nanoparticles via L-in-Lm interfacial polymerization and their pH responsiveness. Colloids Surf. A Physicochem. Eng. Asp. 2020, 603, 125180. [Google Scholar] [CrossRef]
- Kawahara, S.; Klinklai, W.; Kuroda, H.; Isono, Y. Removal of proteins from natural rubber with urea. Polym. Adv. Technol. 2004, 15, 181–184. [Google Scholar] [CrossRef]
- Pukkate, N.; Yamamoto, Y.; Kawahara, S. Mechanism of graft copolymerization of styrene onto deproteinized natural rubber. Colloid Polym. Sci. 2008, 286, 411–416. [Google Scholar] [CrossRef]
- Arayapranee, W.; Prasassarakich, P.; Rempel, G.L. Synthesis of graft copolymers from natural rubber using cumene hydroperoxide redox initiator. J. Appl. Polym. Sci. 2002, 83, 2993–3001. [Google Scholar] [CrossRef]
- Arayapranee, W.; Rempel, G.L. Preparation of a natural rubber core/polymer shell in a nanomatrix by graft copolymerization. J. Appl. Polym. Sci. 2008, 110, 2475–2482. [Google Scholar] [CrossRef]
- Wang, J.; Jia, H. The Effects of Carbon–Silica Dual-Phase Filler on the Crosslink Structure of Natural Rubber. Polymers 2022, 14, 3897. [Google Scholar] [CrossRef]
- Thongnuanchan, B.; Ninjan, R.; Kalkornsurapranee, E.; Lopattananon, N.; Nakason, C. Glutaraldehyde as Ambient Temperature Crosslinking Agent of Latex Films from Natural Rubber Grafted with Poly(diacetone acrylamide). J. Polym. Environ. 2018, 26, 3069–3085. [Google Scholar] [CrossRef]
- Anggaravidya, M.; Amry, A.; Arti, D.K.; Kalembang, E.; Susanto, H.; Hidayat, A.S.; Limansubroto, C.D. Properties of Natural Rubber/Chloroprene Rubber Blend for Rubber Fender Application: Effects of Blend Ratio. Macromol. Symp. 2020, 391, 1900150. [Google Scholar] [CrossRef]
- Kalkornsurapranee, E.; Yung-Aoon, W.; Thongnuanchan, B.; Thitithammawong, A.; Nakason, C.; Johns, J. Influence of grafting content on the properties of cured natural rubber grafted with PMMAs using glutaraldehyde as a cross-linking agent. Adv. Polym. Technol. 2018, 37, 1478–1485. [Google Scholar] [CrossRef]
- Thaptong, P.; Jittham, P.; Sae-oui, P. Effect of conductive carbon black on electrical conductivity and performance of tire tread compounds filled with carbon black/silica hybrid filler. J. Appl. Polym. Sci. 2021, 138, 50855. [Google Scholar] [CrossRef]
- Yimmut, K.; Homchoo, K.; Hinchiranan, N. Poly(butyl acrylate-co-fluorinated acrylate)-graft-natural rubber: Synthesis and application as compatibilizer for natural rubber/poly(butyl acrylate-co-fluorinated acrylate) films. Colloids Surf. A Physicochem. Eng. Asp. 2018, 540, 11–22. [Google Scholar] [CrossRef]
- Longsiri, K.; Mora, P.; Peeksuntiye, W.; Jubsilp, C.; Hemvichian, K.; Karagiannidis, P.; Rimdusit, S. Ultrafine fully vulcanized natural rubber modified by graft-copolymerization with styrene and acrylonitrile monomers. Bioresour. Bioprocess. 2022, 9, 85. [Google Scholar] [CrossRef]
- Song, Y.; Shang, M.; Zhang, H.; Xu, W.; Lu, Q.; Su, Y. Process Characteristics and Rheological Properties of Free Radical Polymerization in Microreactors. Ind. Eng. Chem. Res. 2018, 57, 10922–10934. [Google Scholar] [CrossRef]
- Al Rohily, K.; El-Hamshary, H.; Ghoneim, A.; Modaihsh, A. Controlled Release of Phosphorus from Superabsorbent Phosphate-Bound Alginate-Graft-Polyacrylamide: Resistance to Soil Cations and Release Mechanism. ACS Omega 2020, 5, 32919–32929. [Google Scholar] [CrossRef]
- Liu, X.-J.; Tian, Y.-H.; Lu, Y.-C. A Comparative Study on Emulsion Polymerization Processes of Styrene Initiated by Water-soluble and Oil-soluble Initiators. Chin. J. Polym. Sci. 2019, 37, 142–148. [Google Scholar] [CrossRef]
- Banerjee, A.; Ray, S.K. Nitrocellulose filled and natural rubber grafted poly(styrene-co-acrylonitrile) organophilic membranes for pervaporative recovery of aliphatic alcohols from water. Sep. Purif. Technol. 2021, 266, 118563. [Google Scholar] [CrossRef]
- Lamb, D.J.; Anstey, J.F.; Fellows, C.M.; Monteiro, M.J.; Gilbert, R.G. Modification of natural and artificial polymer colloids by “topology-controlled” emulsion polymerization. Biomacromolecules 2001, 2, 518–525. [Google Scholar] [CrossRef]
- Nakano, T.; Saito, N.; Minami, H. Preparation of Cross-Linked Monodisperse Poly(acrylic acid) Particles by Precipitation Polymerization. Langmuir 2020, 36, 11957–11962. [Google Scholar] [CrossRef] [PubMed]
- Tally, M.; Atassi, Y. Synthesis and characterization of pH-sensitive superabsorbent hydrogels based on sodium alginate-g-poly(acrylic acid-co-acrylamide) obtained via an anionic surfactant micelle templating under microwave irradiation. Polym. Bull. 2016, 73, 3183–3208. [Google Scholar] [CrossRef]
- Ding, B.; Huang, S.; Shen, K.; Hou, J.; Gao, H.; Duan, Y.; Zhang, J. Natural rubber bio-nanocomposites reinforced with self-assembled chitin nanofibers from aqueous KOH/urea solution. Carbohydr. Polym. 2019, 225, 115230. [Google Scholar] [CrossRef] [PubMed]
- Rimdusit, N.; Jubsilp, C.; Mora, P.; Hemvichian, K.; Thuy, T.T.; Karagiannidis, P.; Rimdusit, S. Radiation Graft-Copolymerization of Ultrafine Fully Vulcanized Powdered Natural Rubber: Effects of Styrene and Acrylonitrile Contents on Thermal Stability. Polymers 2021, 13, 3447. [Google Scholar] [CrossRef] [PubMed]
- Nuinu, P.; Srichan, S.; Ngamlerd, A.; Wichian, C.; Prasertsri, S.; Saengsuwan, S.; Hinchiranan, N.; Vudjung, C. Preparation of environment-friendly hydrophilic rubber from natural rubber grafted with sodium acrylate by reactive melt mixing. Polym. Eng. Sci. 2022, 62, 1833–1846. [Google Scholar] [CrossRef]
- Zheng, M.; Lian, F.; Zhu, Y.; Zhang, Y.; Liu, B.; Zhang, L.; Zheng, B. pH-responsive poly (xanthan gum-g-acrylamide-g-acrylic acid) hydrogel: Preparation, characterization, and application. Carbohydr. Polym. 2019, 210, 38–46. [Google Scholar] [CrossRef]
- Saito, T.; Yamano, M.; Nakayama, K.; Kawahara, S. Quantitative analysis of crosslinking junctions of vulcanized natural rubber through rubber-state NMR spectroscopy. Polym. Test. 2021, 96, 107130. [Google Scholar] [CrossRef]
- Silva-Jara, J.; Manríquez-González, R.; López-Dellamary, F.; Puig, J.; Nuño-Donlucas, S. Semi-Continuous Heterophase Polymerization to Synthesize Nanocomposites of Poly(acrylic acid)-Functionalized Carbon Nanotubes. J. Macromol. Sci. Part A 2015, 52, 732–744. [Google Scholar] [CrossRef]
- Kirwan, L.J.; Fawell, P.D.; van Bronswijk, W. In Situ FTIR-ATR Examination of Poly(acrylic acid) Adsorbed onto Hematite at Low pH. Langmuir 2003, 19, 5802–5807. [Google Scholar] [CrossRef]
- Ke, Y.; Wang, Y.; Ren, L.; Lu, L.; Wu, G.; Chen, X.; Chen, J. Photografting polymerization of polyacrylamide on PHBV films (I). J. Appl. Polym. Sci. 2007, 104, 4088–4095. [Google Scholar] [CrossRef]
- Azarian, M.H.; Boochathum, P. Nanofiber films of chloroacetated natural rubber/poly(vinyl alcohol) by electrospinning technique: Silica effects on biodegradation. J. Appl. Polym. Sci. 2018, 135, 46432. [Google Scholar] [CrossRef]
- Lu, M.; He, B.; Wang, L.; Ge, W.; Lu, Q.; Liu, Y.; Zhang, L. Preparation of polystyrene–polyisoprene core–shell nanoparticles for reinforcement of elastomers. Compos. Part B Eng. 2012, 43, 50–56. [Google Scholar] [CrossRef]
- Shin, J.R.; An, G.S.; Choi, S.-C. Influence of Carboxylic Modification Using Polyacrylic Acid on Characteristics of Fe3O4 Nanoparticles with Cluster Structure. Processes 2021, 9, 1795. [Google Scholar] [CrossRef]
- Li, R.; Zhang, C.; Wang, C.; Cheng, Y.; Hu, D. Study on the Mechanism of the Reversible Color Change of Polyacrylic Acid Modified Gold Nanoparticles Responding to pH. Materials 2021, 14, 3679. [Google Scholar] [CrossRef]
- Miedzianowska, J.; Masłowski, M.; Rybiński, P.; Strzelec, K. Modified Nanoclays/Straw Fillers as Functional Additives of Natural Rubber Biocomposites. Polymers 2021, 13, 799. [Google Scholar] [CrossRef] [PubMed]
- Saramolee, P.; Lopattananon, N.; Sahakaro, K. Preparation and some properties of modified natural rubber bearing grafted poly(methyl methacrylate) and epoxide groups. Eur. Polym. J. 2014, 56, 1–10. [Google Scholar] [CrossRef]
- Chen, J.; Liao, L.; Zhang, F.; Gao, T.; Gao, L.; Ma, L.; Ma, X. Improving reinforcement of natural rubber latex by introducing poly-zinc dimethacrylate and sulfur vulcanizing system. Polym. Eng. Sci. 2022, 62, 1549–1561. [Google Scholar] [CrossRef]
- Nuntahirun, P.; Yamamoto, O.; Paoprasert, P. Temperature-responsive N-isopropylacrylamide-grafted natural rubber. Polym. Bull. 2018, 75, 1387–1401. [Google Scholar] [CrossRef]
- Wichaita, W.; Samart, C.; Yoosuk, B.; Kongparakul, S. Cellulose Graft Poly(acrylic acid) and Polyacrylamide: Grafting Efficiency and Heavy Metal Adsorption Performance. Macromol. Symp. 2015, 354, 84–90. [Google Scholar] [CrossRef]
- Lolage, M.; Parida, P.; Gupta, A.; Rautaray, D. Synergistic effects of silica and nanoclay on curing characteristics, processing behaviour and mechanical properties of solution styrene butadiene rubber (SBR)–based tire tread compounds. Emergent Mater. 2022, 5, 957–966. [Google Scholar] [CrossRef]
- Zeng, Z.; Li, Y.; Wang, C.; Zhao, P. The Impact of Nano CaCO3 Modified in situ with Methacrylic Acid on the Structure and Properties of Natural Rubber. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2020, 35, 1169–1176. [Google Scholar] [CrossRef]
- Boonmee, A.; Jarukumjorn, K. Preparation and characterization of silica nanoparticles from sugarcane bagasse ash for using as a filler in natural rubber composites. Polym. Bull. 2020, 77, 3457–3472. [Google Scholar] [CrossRef]
- Borapak, W.; Chueangchayaphan, N.; Pichaiyut, S.; Chueangchayaphan, W. Cure characteristics and physico-mechanical properties of natural rubber/silica composites: Effect of natural rubber-graft-poly(2-hydroxyethyl acrylate) content. Polym. Bull. 2021, 78, 2009–2023. [Google Scholar] [CrossRef]
- Mei, J.; Geng, H.; Yu, H.; Shi, J.; Zhao, Y.; Yu, R.; Liao, J.; Chen, Y. Preparation and performance of oleylamine modified silica-reinforced natural rubber composites. J. Appl. Polym. Sci. 2021, 138, 49907. [Google Scholar] [CrossRef]
- Wang, X.; Yao, F.; Su, J.; Zhang, X.; Tong, X.; Qin, Z.; Yuan, C. Modification of Natural Rubber Latex by Graft Copolymerization of 2-Ethylhexyl Acrylate and Methacrylic Acid. Trans. Tianjin Univ. 2020, 26, 314–323. [Google Scholar] [CrossRef]
- Chueangchayaphan, W.; Chueangchayaphan, N.; Tanrattanakul, V.; Muangsap, S. Influences of the grafting percentage of natural rubber-graft-poly(2-hydroxyethyl acrylate) on properties of its vulcanizates. Polym. Int. 2018, 67, 739–746. [Google Scholar] [CrossRef]
- Ghorai, S.; Mondal, D.; Hait, S.; Ghosh, A.K.; Wiessner, S.; Das, A.; De, D. Devulcanization of Waste Rubber and Generation of Active Sites for Silica Reinforcement. ACS Omega 2019, 4, 17623–17633. [Google Scholar] [CrossRef] [Green Version]
- Ruamcharoen, J.; Chotisuwan, S.; Ruamcharoen, P. Tensile Properties and Morphology of Natural Rubber-Kaolinite Organoclay Composites. Adv. Mater. Res. 2012, 488–489, 701–705. [Google Scholar] [CrossRef]
- Nooma, S.; Magaraphan, R. Core–shell natural rubber and its effect on toughening and mechanical properties of poly(methyl methacrylate). Polym. Bull. 2019, 76, 3329–3354. [Google Scholar] [CrossRef]
- Luo, C.; Wu, X.; Zhang, T.; Chi, S.-S.; Liu, Z.; Wang, J.; Wang, C.; Deng, Y. A Four-Armed Polyacrylic Acid Homopolymer Binder with Enhanced Performance for SiOx/Graphite Anode. Macromol. Mater. Eng. 2021, 306, 2000525. [Google Scholar] [CrossRef]
- Permyakova, N.; Zheltonozhskaya, T.; Revko, O.; Grischenko, L. Self-Assembly and Metalation of pH-Sensitive Double Hydrophilic Block Copolymers with Interacting Polymer Components. Macromol. Symp. 2012, 317–318, 63–74. [Google Scholar] [CrossRef]
- Kunitskaya, L.; Zheltonozhskaya, T.; Destarac, M.; Mazieres, S. Block copolymers containing polyacrylamide and polyacrylic acid: Bulk structure and hydrogen bonds system. Mol. Cryst. Liq. Cryst. 2017, 642, 89–98. [Google Scholar] [CrossRef]
- Tangudom, P.; Thongsang, S.; Sombatsompop, N. Cure and mechanical properties and abrasive wear behavior of natural rubber, styrene–butadiene rubber and their blends reinforced with silica hybrid fillers. Mater. Des. 2014, 53, 856–864. [Google Scholar] [CrossRef]
- Roy, K.; Debnath, S.C.; Raengthon, N.; Potiyaraj, P. Understanding the reinforcing efficiency of waste eggshell-derived nano calcium carbonate in natural rubber composites with maleated natural rubber as compatibilizer. Polym. Eng. Sci. 2019, 59, 1428–1436. [Google Scholar] [CrossRef]
- Sowińska-Baranowska, A.; Maciejewska, M. Influence of the Silica Specific Surface Area and Ionic Liquids on the Curing Characteristics and Performance of Styrene–Butadiene Rubber Composites. Materials 2021, 14, 5302. [Google Scholar] [CrossRef]
- Peng, S.; Iroh, J.O. Dependence of the Dynamic Mechanical Properties and Structure of Polyurethane-Clay Nanocomposites on the Weight Fraction of Clay. J. Compos. Sci. 2022, 6, 173. [Google Scholar] [CrossRef]
- Patidar, D.; Agrawal, S.; Saxena, N.S. Storage modulus and glass transition behaviour of CdS/PMMA nanocomposites. J. Exp. Nanosci. 2011, 6, 441–449. [Google Scholar] [CrossRef]

















| Ingredients | Content (phr) | |
|---|---|---|
| P10-DPNR | P30-DPNR | |
| DPNR | 100 | 100 |
| Terric16A | 5 | 5 |
| CHP | 1 | 1 |
| TEPA | 1 | 1 |
| Acrylic acid | 5 | 15 |
| Acrylamide | 5 | 15 |
| Samples | Content (phr) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| DPNR | DPNR- 10Si | DPNR- 20Si | P10-DPNR | P10-DPNR-10Si | P10-DPNR-20Si | P30-DPNR | P30-DPNR-10Si | P30-DPNR- 20Si | |
| DPNR or modified DPNR | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Silica | 0 | 10 | 20 | 0 | 10 | 20 | 0 | 10 | 20 |
| ZnO | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Steric acid | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| CBS | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| DPG | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Sulfur | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 |
| No | Samples | Monomer Content (phr) | Particle Size (µm) | Zeta Potential (mV) | Tg 2 (°C) | |
|---|---|---|---|---|---|---|
| SEM 1 | DLS | |||||
| 1 | DPNR | 0 | 0.384 ± 0.089 | 0.610 ± 0.078 | −20.1 | −65.0 |
| 2 | P10-DPNR | 10 | 2.086 ± 0.506 | 2.533 ± 0.080 | −33.2 | −64.0 |
| 3 | P30-DPNR | 30 | 0.805 ± 0.222 | 0.874 ± 0.005 | −64.5 | −62.9 |
| No | Samples | Natural Rubber (phr) | Silica (phr) | Ts2 (min) | Tc90 (min) | ML (dNm) | MH (dNm) | MH-ML (dNm) | CRI (min−1) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | DPNR | 100 | 0 | 1.55 | 3.71 | 0.20 | 8.71 | 8.51 | 46.29 |
| 2 | DPNR-10Si | 100 | 10 | 2.50 | 6.22 | 0.30 | 8.82 | 8.52 | 26.88 |
| 3 | DPNR-20Si | 100 | 20 | 3.24 | 6.45 | 0.36 | 9.57 | 9.21 | 31.15 |
| 4 | P10-DPNR | 100 | 0 | 0.91 | 5.35 | 0.50 | 9.99 | 9.49 | 22.52 |
| 5 | P10-DPNR-10Si | 100 | 10 | 1.61 | 3.62 | 0.50 | 11.05 | 10.55 | 49.75 |
| 6 | P10-DPNR-20Si | 100 | 20 | 2.02 | 3.94 | 0.60 | 12.78 | 12.18 | 52.08 |
| 7 | P30-DPNR | 100 | 0 | 1.16 | 4.05 | 0.50 | 10.95 | 10.45 | 34.60 |
| 8 | P30-DPNR-10Si | 100 | 10 | 1.33 | 3.26 | 0.55 | 12.51 | 11.96 | 51.81 |
| 9 | P30-DPNR-20Si | 100 | 20 | 1.35 | 4.67 | 1.37 | 19.16 | 17.79 | 30.12 |
| Samples | DPNR | DPNR-10Si | P10-DPNR | P10-DPNR-10Si | P10-DPNR-20Si | P30-DPNR |
|---|---|---|---|---|---|---|
| Tg (°C) | −48.06 | −49.98 | −47.87 | −45.47 | −45.49 | −47.60 |
| Tan δ peak height | 2.73 | 2.33 | 2.65 | 2.18 | 1.98 | 1.26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inphonlek, S.; Bureewong, N.; Jarukumjorn, K.; Chumsamrong, P.; Ruksakulpiwat, C.; Ruksakulpiwat, Y. Preparation of Poly(acrylic acid-co-acrylamide)-Grafted Deproteinized Natural Rubber and Its Effect on the Properties of Natural Rubber/Silica Composites. Polymers 2022, 14, 4602. https://doi.org/10.3390/polym14214602
Inphonlek S, Bureewong N, Jarukumjorn K, Chumsamrong P, Ruksakulpiwat C, Ruksakulpiwat Y. Preparation of Poly(acrylic acid-co-acrylamide)-Grafted Deproteinized Natural Rubber and Its Effect on the Properties of Natural Rubber/Silica Composites. Polymers. 2022; 14(21):4602. https://doi.org/10.3390/polym14214602
Chicago/Turabian StyleInphonlek, Supharat, Namthip Bureewong, Kasama Jarukumjorn, Pranee Chumsamrong, Chaiwat Ruksakulpiwat, and Yupaporn Ruksakulpiwat. 2022. "Preparation of Poly(acrylic acid-co-acrylamide)-Grafted Deproteinized Natural Rubber and Its Effect on the Properties of Natural Rubber/Silica Composites" Polymers 14, no. 21: 4602. https://doi.org/10.3390/polym14214602
APA StyleInphonlek, S., Bureewong, N., Jarukumjorn, K., Chumsamrong, P., Ruksakulpiwat, C., & Ruksakulpiwat, Y. (2022). Preparation of Poly(acrylic acid-co-acrylamide)-Grafted Deproteinized Natural Rubber and Its Effect on the Properties of Natural Rubber/Silica Composites. Polymers, 14(21), 4602. https://doi.org/10.3390/polym14214602