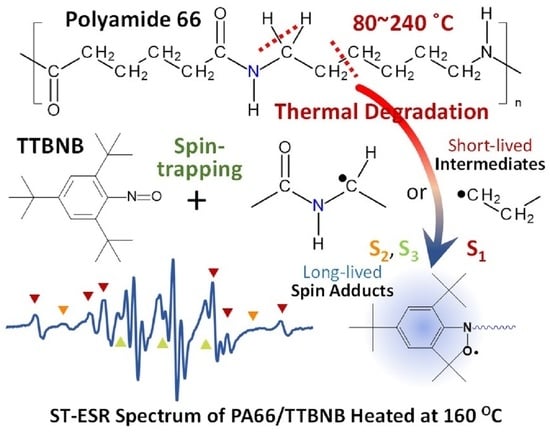
Spin-Trapping Analysis of the Thermal Degradation Reaction of Polyamide 66
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Characterization
2.3.1. TG-DTA Measurements
2.3.2. GPC Measurements
2.3.3. FTIR Measurements
2.3.4. ESR Measurements
3. Results and Discussion
3.1. TG-DTA of PA66
3.2. Thermal Degradation Analysis with GPC
3.3. Thermal Degradation Analysis with FTIR
3.4. Radical Analysis by Spin-Trapping ESR
3.4.1. ESR Measurements
3.4.2. Assignments of Spin Adducts
| Observed Spin Adducts in This Paper | Referred Assignments in Previous Articles | |||||||
|---|---|---|---|---|---|---|---|---|
| Assigned Molecular Structure | hfcc and g | Trapped Radical | hfcc and g | Materials | Ref. | |||
| S1 |  | aH(2) | 1.75 | ●CH2CH2− | aH(2) | 1.799 | (n-Bu)3Sn-Br(or I) /TTBNB/benzene | [30] |
| aN | 1.40 | aN | 1.346 | |||||
| aHm | 0.08 | aHm | 0.083 | |||||
| g | 2.0053 | g | 2.0060 | |||||
| S2 |  | aH(1) | 2.17 | −●CH−CH2− | aH(1) | 2.16 | n-C12C26(paraffin) /TTBNB | [32] |
| aN | 1.43 | aN | 1.33 | |||||
| aHm | 0.08 | aHm | N/A | |||||
| g | 2.0046 | g | 2.0061 | |||||
| S3 |  | aH(1) | 0.20 | −CH2−●CH−CH2− | aH(1) | 0.18 | C6H12/(tert-BuO)2/TTBNB/benzene | [30] |
| aN | 1.00 | aN | 1.10 | |||||
| aHm | 0.20 | aHm | 0.18 | |||||
| g | 2.0039 | g | 2.0036 ~ 2.0040 | |||||
| S4 |  | aN | 1.02 | ●C(CH3)3(TTBNB) | aN | 1.05 | n-eicosane /TTBNB | [33] |
| aHm | 0.17 | aHm | 0.193 | |||||
| g | 2.0033 | g | 2.0046 | |||||
| S5 |  | aH(2) | 1.42 | ●CH2− (TTBNB) | aH(2) | 1.46 | PP/TTBNB | [16] |
| aN | 1.29 | aN | 1.05 | |||||
| aHm | 0.08 | aHm | 0.10 | |||||
| g | 2.0052 | g | 2.0054 | |||||
| S6 | unassinged | aH | N.A. | aH | - | N.A. | ||
| aN | 1.3~1.5 | aN | - | |||||
| aHm | N.A. | aHm | - | |||||
| g | 2.0033 | g | - | |||||
3.5. Reaction Mechanism of PA66 Thermal Degradation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef]
- Andrady, A.L. The plastic in microplastics: A review. Mar. Pollut. Bull. 2017, 119, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Isobe, A.; Iwasaki, S.; Uchida, K.; Tokai, T. Abundance of non-conservative microplastics in the upper ocean from 1957 to 2066. Nat. Commun. 2019, 10, 417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halle, A.; Ladirat, L.; Martignac, M.; Mingotaud, A.F.; Boyron, O.; Perez, E. To what extent are microplastics from the open ocean weathered? Environ. Pollut. 2017, 227, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Lambert, S.; Wagner, M. Formation of microscopic particles during the degradation of different polymers. Chemosphere 2016, 161, 510–517. [Google Scholar] [CrossRef]
- Naveed, K.-R.; Wang, L.; Yu, H.; Ullah, R.S.; Haroon, M.; Fahad, S.; Li, J.; Elshaarani, T.; Khan, R.U.; Nazir, A. Recent progress in the electron paramagnetic resonance study of polymers. Polym. Chem. 2018, 9, 3306–3335. [Google Scholar] [CrossRef]
- Rånby, B.; Rabek, J.F. ESR Spectroscopy in Polymer Research; Springer: Berlin/Heidelberg, Germany, 1977; Volume 1. [Google Scholar]
- Schlick, S. (Ed.) Advanced ESR Methods in Polymer Research; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006. [Google Scholar]
- Buettner, G.R. Spin Trapping: ESR parameters of spin adducts 1474 1528V. Free Radic. Biol. Med. 1987, 3, 259–303. [Google Scholar] [CrossRef]
- Janzen, E.G.; Haire, D.L. ChemInform Abstract: Two Decades of Spin Trapping. ChemInform 1991, 22. [Google Scholar] [CrossRef]
- Janzen, E.G.; Stronks, H.J.; Dubose, C.M.; Poyer, J.L.; McCay, P.B. Chemistry and Biology of Spin-Trapping Radicals Associated with Halocarbon Metabolism In Vitro and In Vivo. Environ. Health Perspect. 1985, 64, 151–170. [Google Scholar] [CrossRef]
- Janzen, E.G. Spin trapping. Acc. Chem. Res. 1971, 4, 31–40. [Google Scholar] [CrossRef]
- Sono, M.; Kinashi, K.; Sakai, W.; Tsutsumi, N. Spin-trapping analysis of thermal degradation reaction of poly(butylene terephthalate). Macromolecules 2017, 50, 254–263. [Google Scholar] [CrossRef]
- Sono, M.; Kinashi, K.; Sakai, W.; Tsutsumi, N. Spin-Trapping Analysis and Characterization of Thermal Degradation of Thermoplastic Poly(ether-ester) Elastomer. Macromolecules 2018, 51, 1088–1099. [Google Scholar] [CrossRef]
- Hayashi, T.; Kinashi, K.; Sakai, W.; Tsutsumi, N.; Fujii, A.; Inada, S.; Yamamoto, H. Spin-trapping analysis for thermal degradation of poly(vinyl alcohol). Polymer 2021, 217, 123416. [Google Scholar] [CrossRef]
- Nguyen, T.A.; Ichise, S.; Kinashi, K.; Sakai, W.; Tsutsumi, N.; Okubayashi, S. Spin trapping analysis of the thermal degradation of polypropylene. Polym. Degrad. Stab. 2022, 197, 109871. [Google Scholar] [CrossRef]
- Levchik, S.V.; Weil, E.D.; Lewin, M. Thermal decomposition of aliphatic nylons. Polym. Int. 1999, 48, 532–557. [Google Scholar] [CrossRef]
- Pielichowski, K.; Njuguna, J. Thermal Degradation of Polymeric Materials; EngineeringPro Collection; iSmithers Rapra Publishing: Shropshire, UK, 2005. [Google Scholar]
- Holland, B.J.; Hay, J.N. Thermal degradation of nylon polymers. Polym. Int. 2000, 49, 943–948. [Google Scholar] [CrossRef]
- Schaffer, M.A.; Marchildon, E.K.; McAuley, K.B.; Cunningham, M.F. Thermal Nonoxidative Degradation of Nylon 6,6. Macromol. Sci. Part C Polym. Rev. 2000, C40, 233–272. [Google Scholar] [CrossRef]
- Ballistreri, A.; Garozzo, D.; Giuffrida, M.; Montaudo, G. Mechanism of thermal decomposition of Nylon 66. Macromolecules 1987, 20, 2991–2997. [Google Scholar] [CrossRef]
- Herrera, M.; Matuschek, G.; Kettrup, A. Main products and kinetics of the thermal degradation of polyamides. Chemosphere 2001, 42, 601–607. [Google Scholar] [CrossRef]
- Gonçalves, E.S.; Poulsen, L.; Ogilby, P.R. Mechanism of the temperature-dependent degradation of polyamide 66 films exposed to water. Polym. Degrad. Stab. 2007, 92, 1977–1985. [Google Scholar] [CrossRef]
- El-Mazry, C.; Correc, O.; Colin, X. A new kinetic model for predicting polyamide 6-6 hydrolysis and its mechanical embrittlement. Polym. Degrad. Stab. 2012, 97, 1049–1059. [Google Scholar] [CrossRef] [Green Version]
- El-Mazry, C.; Hassine, M.B.; Correc, O.; Colin, X. Thermal oxidation kinetics of additive free polyamide 6-6. Polym. Degrad. Stab. 2013, 98, 22–36. [Google Scholar] [CrossRef] [Green Version]
- Catiker, E.; Guven, O.; Ozarslan, O.; Chipara, M. ESR investigations on γ-ray irradiated 3-methyl nylon 3. Nucl. Instrum. Methods Phys. Res. B. 2008, 266, 3100–3106. [Google Scholar] [CrossRef]
- Pagrk, J.B.; Devries, K.L.; Statton, W.O. Chain rupture during tensile deformation of nylon 6 fibers. J. Macromol.Sci. Part B Phys. 1978, 15, 205–227. [Google Scholar] [CrossRef]
- Kausch-Blecken Von Schmeling, H.H. Application of Electron Spin Resonance Techniques to High Polymer Fracture. J. Macromol. Sci. Part C: Polym. Rev. 1970, 4, 243–280. [Google Scholar] [CrossRef]
- Lee, S.S.; Phillips, P.J. Melt crystallized polyamide 6.6 and its copolymers, Part, I. Melting point—Lamellar thickness relations in the homopolymer. Eur. Polym. J. 2007, 43, 1933–1951. [Google Scholar] [CrossRef]
- Konaka, R.; Terabe, S. Spin trapping of short-lived free radicals by use of 2,4,6-tri-tert-butylnitrosobenzene. J. Am. Chem. Soc. 1971, 93, 4306–4307. [Google Scholar] [CrossRef]
- Yamada, B.; Fujity, M.; Sakamoto, K.; Otsu, T. Spin trapping of propagating radicals with 2,4,6-tri-tert-butyl-1-nitrosobenzene in radical polymerization initiated with di-tert-butyl hyponitrite. Polym. Bull. 1994, 33, 309–316. [Google Scholar] [CrossRef]
- Qu, B.J.; Xu, Y.H.; Shi, W.F.; Ránby, B. Photoinitiated Cross-Linking of Low-Density Polyethylene. 7. Initial Radical Reactions with Model Compounds Studied by Spin-Trapping ESR Spectroscopy. Macromolecules 1992, 25, 5220–5224. [Google Scholar] [CrossRef]
- Tabata, M.; Sohma, J.; Yamaoka, H.; Matsuyama, T. A spin-trapping study on crosslinks induced by γ-and neutron irradiations of n-eicosane. Inter. J. Rad. App. Inst. Part C. Rad. Phys. Chem. 1986, 27, 35–39. [Google Scholar] [CrossRef]
- Richaud, E.; Diogo, O.O.; Fayolle, B.; Verdu, J.; Guilment, J.; Fernagut, F. Review: Auto-oxidation of aliphatic polyamides. Polym. Degrad. Stab. 2013, 98, 1929–1939. [Google Scholar] [CrossRef] [Green Version]
- Arash, B.; Thijsse, B.J.; Pecenko, A.; Simone, A. Effect of water content on the thermal degradation of amorphous polyamide 6,6: A collective variable-driven hyperdynamics study. Polym. Degrad. Stab. 2017, 146, 260–266. [Google Scholar] [CrossRef]








| Condition | Mn | Mw | Mw/Mn |
|---|---|---|---|
| Not heated | 15,600 | 43,600 | 2.79 |
| After heating | 14,200 | 43,500 | 3.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurima, A.; Kinashi, K.; Sakai, W.; Tsutsumi, N. Spin-Trapping Analysis of the Thermal Degradation Reaction of Polyamide 66. Polymers 2022, 14, 4748. https://doi.org/10.3390/polym14214748
Kurima A, Kinashi K, Sakai W, Tsutsumi N. Spin-Trapping Analysis of the Thermal Degradation Reaction of Polyamide 66. Polymers. 2022; 14(21):4748. https://doi.org/10.3390/polym14214748
Chicago/Turabian StyleKurima, Akihiro, Kenji Kinashi, Wataru Sakai, and Naoto Tsutsumi. 2022. "Spin-Trapping Analysis of the Thermal Degradation Reaction of Polyamide 66" Polymers 14, no. 21: 4748. https://doi.org/10.3390/polym14214748
APA StyleKurima, A., Kinashi, K., Sakai, W., & Tsutsumi, N. (2022). Spin-Trapping Analysis of the Thermal Degradation Reaction of Polyamide 66. Polymers, 14(21), 4748. https://doi.org/10.3390/polym14214748