Study on Degradation of 1,2,4-TrCB by Sugarcane Cellulose-TiO2 Carrier in an Intimate Coupling of Photocatalysis and Biodegradation System
Abstract
:1. Introduction
2. Material and Methods
2.1. Preparing Materials
2.2. Methods
- (1)
- Experimental methods
- (2)
- Analytical methods
3. Results and Discussion
3.1. Degradation of 1,2,4-TrCB in ICPB
3.2. Mineralization of 1,2,4-TrCB in ICPB
3.3. Construction of Free Radicals in ICPB
3.4. Degradation Pathway of 1,2,4-TrCB in ICPB
3.5. Microbial Community Response Analysis
3.5.1. SEM Analysis of Microorganisms in Carriers
3.5.2. Genera Composition of Microbial Community
3.5.3. Correlation Analysis of Microbial Community
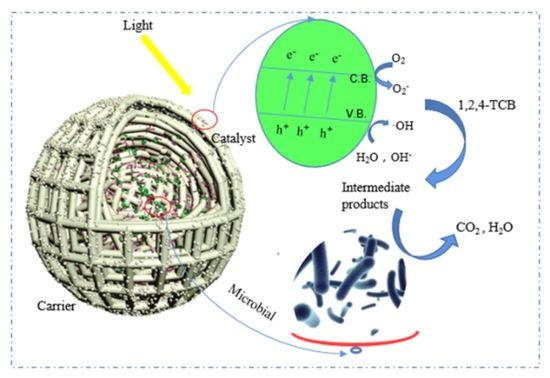
3.6. Degradation Mechanism of 1,2,4-TrCB in ICPB
4. Conclusions
- (1)
- The ICPB system was constructed using a sugarcane cellulose-TiO2 carrier. This technology played an important role in promoting the degradation of 1,2,4-TrCB. Compared with biodegradation and photocatalysis alone, the removal rates of 1,2,4-TrCB increased by 68.01% and 14.81%, respectively, and the mineralization rates increased by 50.30% and 11.50%, respectively.
- (2)
- The sugarcane cellulose-TiO2 carrier protected the dominant bacteria in the biofilm from damage by photocatalysis. The microorganism decomposed some of the photocatalysis products, making more free radicals that were used for the degradation of the intermediate products, thus improving the degradation rate and mineralization rate of 1,2,4-TrCB.
Author Contributions
Funding
Conflicts of Interest
References
- Shang, X.; Yang, L.; Ouyang, D.; Zhang, B.; Zhang, W.; Gu, M.; Li, J.; Chen, M.; Huang, L.; Qian, L. Enhanced removal of 1,2,4-trichlorobenzene by modified biochar supported nanoscale zero-valent iron and palladium. Chemosphere 2020, 249, 126518. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Liu, L.; Wang, F.; Liu, X.; Huang, Z.; Zhao, H.; Qi, B.; Zhang, G. Exogenous silicon enhances resistance to 1,2,4-trichlorobenzene in rice. Sci. Total Environ. 2022, 845, 157248. [Google Scholar] [CrossRef]
- Conte, L.O.; Dominguez, C.M.; Checa-Fernandez, A.; Santos, A. Vis LED Photo-Fenton Degradation of 124-Trichlorobenzene at a Neutral pH Using Ferrioxalate as Catalyst. Int. J. Environ. Res. Public Health 2022, 19, 9733. [Google Scholar] [CrossRef] [PubMed]
- Egorova, D.O.; Nazarova, E.A.; Demakov, V.A. New Lindane-Degrading Strains Achromobacter sp. NE1 and Brevundimonas sp. 242. Microbiology 2021, 90, 392–396. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, N.; Qiao, W.; Ye, S. Effects of o-nitro-p-methylphenol and o-amino-p-methylphenol on the anaerobic biodegradation of 1,2,4-TCB. Earth Sci. Front. 2021, 28, 159–166. [Google Scholar]
- Qian, Y.; Kong, X. Research Progresses in Environmental Remediation Technologies for 1,2,4-Trichlorobenzene Pollution. Environ. Prot. Chem. Ind. 2015, 35, 147–153. [Google Scholar]
- Marsolek, M.D.; Torres, C.I.; Hausner, M.; Rittmann, B.E. Intimate coupling of photocatalysis and biodegradation in a photocatalytic circulating-bed biofilm reactor. Biotechnol. Bioeng. 2008, 101, 83–92. [Google Scholar] [CrossRef]
- Ma, D.; Zou, D.; Zhou, D.; Li, T.; Dong, S.; Xu, Z.; Dong, S. Phenol removal and biofilm response in coupling of visible-light-driven photocatalysis and biodegradation: Effect of hydrothermal treatment temperature. Int. Biodeterior. Biodegrad. 2015, 104, 178–185. [Google Scholar] [CrossRef]
- Xiong, H.; Zou, D.; Zhou, D.; Dong, S.; Wang, J.; Rittmann, B.E. Enhancing degradation and mineralization of tetracycline using intimately coupled photocatalysis and biodegradation (ICPB). Chem. Eng. J. 2017, 316, 7–14. [Google Scholar] [CrossRef]
- Zhang, C.; Fu, L.; Xu, Z.; Xiong, H.; Zhou, D.; Huo, M. Contrasting roles of phenol and pyrocatechol on the degradation of 4-chlorophenol in a photocatalytic-biological reactor. Environ. Sci. Pollut. Res. 2017, 24, 24725–24731. [Google Scholar] [CrossRef]
- Lu, Z.; Xu, Y.; Akbari, M.Z.; Liang, C.; Peng, L. Insight into integration of photocatalytic and microbial wastewater treatment technologies for recalcitrant organic pollutants: From sequential to simultaneous reactions. Chemosphere 2022, 295, 133952. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Lan, X.; Shi, J.; Wang, L. Loofah sponge as an environment-friendly biocarrier for intimately coupled photocatalysis and biodegradation (ICPB). J. Water Process Eng. 2021, 40, 101965. [Google Scholar] [CrossRef]
- Zhou, D.; Xu, Z.; Dong, S.; Huo, M.; Dong, S.; Tian, X.; Cui, B.; Xiong, H.; Li, T.; Ma, D. Intimate Coupling of Photocatalysis and Biodegradation for Degrading Phenol Using Different Light Types: Visible Light vs. UV Light. Environ. Sci. Technol. 2015, 49, 7776–7783. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xing, Z.; Zhang, H.; Li, Z.; Wu, X.; Zhang, X.; Zhang, Y.; Zhou, W. High thermostable ordered mesoporous SiO2-TiO2 coated circulating-bed biofilm reactor for unpredictable photocatalytic and biocatalytic performance. Appl. Catal. B Environ. 2016, 180, 521–529. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, C.; Shuai, D.; Naraginti, S.; Wang, D.; Zhang, W. Visible-light-driven photocatalytic inactivation of MS2 by metal-free g-C3N4: Virucidal performance and mechanism. Water Res. 2016, 106, 249–258. [Google Scholar] [CrossRef]
- Wang, C.; Ren, B.; Hursthouse, A.S.; Hou, B.; Peng, Y. Visible Light-Driven Photocatalytic Degradation of 1,2,4-trichlorobenzene with Synthesized Co3O4 Photocatalyst. Pol. J. Environ. Stud. 2018, 27, 2285–2292. [Google Scholar] [CrossRef]
- Kozhevnikova, N.S.; Gorbunova, T.I.; Vorokh, A.S.; Pervova, M.G.; Zapevalov, A.Y.; Saloutin, V.I.; Chupakhin, O.N. Nanocrystalline TiO2 doped by small amount of pre-synthesized colloidal CdS nanoparticles for photocatalytic degradation of 1,2,4-trichlorobenzene. Sustain. Chem. Pharm. 2019, 11, 1–11. [Google Scholar] [CrossRef]
- Zhang, W.; Shi, Z.; Zhang, Q.; Zhang, H.; Xu, S.-K. Photocatalytic degradation of 1,2, 4-trichlorobenzene with TiO2 coated on carbon nanotubes. Huan Jing Ke Xue = Huanjing Kexue 2011, 32, 1974–1979. [Google Scholar]
- Shi, Z.; Zhang, Q.; Zhang, W.; Xu, S.-K.; Zhang, H. Study on photocatalytic degradation of 1, 2, 3-trichlorobenzene using the microwaved MWNTs/TiO2 composite. Huan Jing Ke Xue = Huanjing Kexue 2012, 33, 3840–3846. [Google Scholar]
- Brunsbach, F.R.; Reineke, W. Degradation of chlorobenzenes in soil slurry by a specialized organism. Appl. Microbiol. Biotechnol. 1994, 42, 415–420. [Google Scholar] [CrossRef]
- Dermietzel, J.; Vieth, A. Chloroaromatics in groundwater: Chances of bioremediation. Environ. Geol. 2002, 41, 683–689. [Google Scholar]
- Dong, W.H.; Zhang, P.; Lin, X.Y.; Zhang, Y.; Taboure, A. Natural attenuation of 1,2,4-trichlorobenzene in shallow aquifer at the Luhuagang’s landfill site, Kaifeng, China. Sci. Total Environ. 2015, 505, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Mao, W.; Zhang, L.; Wang, T.; Bai, Y.; Guan, Y. Fabrication of highly efficient Bi2WO6/CuS composite for visible-light photocatalytic removal of organic pollutants and Cr(VI) from wastewater. Front. Environ. Sci. Eng. 2021, 15, 52. [Google Scholar] [CrossRef]
- Amini Herab, A.; Salari, D.; Tseng, H.-H.; Niaei, A.; Mehrizadeh, H.; Rahimi Aghdam, T. Synthesis of BiFeO3 nanoparticles for the photocatalytic removal of chlorobenzene and a study of the effective parameters. React. Kinet. Mech. Catal. 2020, 131, 437–452. [Google Scholar] [CrossRef]
- Pan, X.; Wei, J.; Qu, R.; Xu, S.; Chen, J.; Al-Basher, G.; Li, C.; Shad, A.; Dar, A.A.; Wang, Z. Alumina-mediated photocatalytic degradation of hexachlorobenzene in aqueous system: Kinetics and mechanism. Chemosphere 2020, 257, 127256. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Xing, L. Adsorption and Degradation of 1, 2, 4-Trichlorobenzene by Activated Carbon-Microorganisms in Soil. J. Agro-Environ. Sci. 2015, 34, 1535–1541. [Google Scholar]
- Zhang, Y.; Wang, L.; Rittmann, B.E. Integrated photocatalytic-biological reactor for accelerated phenol mineralization. Appl. Microbiol. Biotechnol. 2010, 86, 1977–1985. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, X.; Chen, L.; Rittmann, B.E. Integrated photocatalytic-biological reactor for accelerated 2,4,6-trichlorophenol degradation and mineralization. Biodegradation 2012, 23, 189–198. [Google Scholar] [CrossRef]
- Wen, D.; Li, G.; Xing, R.; Park, S.; Rittmann, B.E. 2,4-DNT removal in intimately coupled photobiocatalysis: The roles of adsorption, photolysis, photocatalysis, and biotransformation. Appl. Microbiol. Biotechnol. 2012, 95, 263–272. [Google Scholar] [CrossRef]
- Korosi, L.; Bognar, B.; Bouderias, S.; Castelli, A.; Scarpellini, A.; Pasquale, L.; Prato, M. Highly-efficient photocatalytic generation of superoxide radicals by phase-pure rutile TiO2 nanoparticles for azo dye removal. Appl. Surf. Sci. 2019, 493, 719–728. [Google Scholar] [CrossRef]
- Liu, X.; Kong, L.; Hujiabudula, M.; Xu, S.; Ma, F.; Zhong, M.; Liu, J.; Abulikemu, A. Progress in preparation and photocatalytic properties of molybdate. Appl. Chem. Ind. 2021, 50, 217–224. [Google Scholar]
- Sobczynski, A.; Dobosz, A. Water purification by photocatalysis on semiconductors. Pol. J. Environ. Stud. 2001, 10, 195–205. [Google Scholar]
- Luan, Y.; Jing, L.; Meng, Q.; Nan, H.; Luan, P.; Xie, M.; Feng, Y. Synthesis of Efficient Nanosized Rutile TiO2 and Its Main Factors Determining Its Photodegradation Activity: Roles of Residual Chloride and Adsorbed Oxygen. J. Phys. Chem. C 2012, 116, 17094–17100. [Google Scholar] [CrossRef]
- Lichtenberger, J.; Amiridis, M.D. Catalytic oxidation of chlorinated benzenes over V2O5/TiO2 catalysts. J. Catal. 2004, 223, 296–308. [Google Scholar] [CrossRef]
- Lin, S.; Su, G.; Zheng, M.; Jia, M.; Qi, C.; Li, W. The degradation of 1,2,4-trichlorobenzene using synthesized Co3O4 and the hypothesized mechanism. J. Hazard. Mater. 2011, 192, 1697–1704. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Zhang, X.; Ma, M.; Wang, L.; Xue, Z.; Hou, Y.; Ye, Z.; Liu, T. 1,2,4-trichlorobenzene decomposition using non-thermal plasma technology. Plasma Sci. Technol. 2020, 22, 034011. [Google Scholar] [CrossRef]
- Feng, Q.; Wang, Q.; Yu, Y.; Jing, Z. Catalytic Degradation of 1,2,4-Trichlorobenzene with Co-Mn Polyhedral Composite Oxides by Sol-Gel Process. Chem. World 2017, 58, 7–12. [Google Scholar]
- Zhang, W.; Li, L.; Zhang, Q.; Xu, S.; Zhang, H. Study on photocatalytic degradation of typical chlorobenzenes with MWNTs/TiO2. Acta Sci. Circumstantiae 2012, 32, 631–638. [Google Scholar]
- Doronina, N.V.; Kaparullina, E.N.; Trotsenko, Y.A. Methyloversatilis thermotolerans sp nov. a novel thermotolerant facultative methylotroph isolated from a hot spring. Int. J. Syst. Evol. Microbiol. 2014, 64, 158–164. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.; Kalyuzhnaya, M.; Chandran, K. Comparative proteomic analysis reveals insights into anoxic growth of Methyloversatilis universalis FAM5 on methanol and ethanol. Environ. Microbiol. 2012, 14, 2935–2945. [Google Scholar] [CrossRef]
- Smalley, N.E.; Taipale, S.; De Marco, P.; Doronina, N.V.; Kyrpides, N.; Shapiro, N.; Woyke, T.; Kalyuzhnaya, M.G. Functional and genomic diversity of methylotrophic Rhodocyclaceae: Description of Methyloversatilis discipulorum sp nov. Int. J. Syst. Evol. Microbiol. 2015, 65, 2227–2233. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhou, L.; Lin, H.; Zhang, W.; Xia, S. Nitrate effects on perchlorate reduction in a H2/CO2-based biofilm. Sci. Total Environ. 2019, 694, 133564. [Google Scholar] [CrossRef] [PubMed]
- Kittichotirat, W.; Good, N.M.; Hall, R.; Bringel, F.; Lajus, A.; Medigue, C.; Smalley, N.E.; Beck, D.; Bumgarner, R.; Vuilleumier, S.; et al. Genome Sequence of Methyloversatilis universalis FAM5T, a Methylotrophic Representative of the Order Rhodocyclales. J. Bacteriol. 2011, 193, 4541–4542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rochman, F.F.; Sheremet, A.; Tamas, I.; Saidi-Mehrabad, A.; Kim, J.-J.; Dong, X.; Sensen, C.W.; Gieg, L.M.; Dunfield, P.F. Benzene and Naphthalene Degrading Bacterial Communities in an Oil Sands Tailings Pond. Front. Microbiol. 2017, 8, 1845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, Z.; Wei, Z. Microbial flora analysis for the degradation of beta-cypermethrin. Environ. Sci. Pollut. Res. 2017, 24, 6554–6562. [Google Scholar] [CrossRef]
- Jiao, S.; Liu, Z.; Lin, Y.; Yang, J.; Chen, W.; Wei, G. Bacterial communities in oil contaminated soils: Biogeography and co-occurrence patterns. Soil Biol. Biochem. 2016, 98, 64–73. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Lin, H.; Yin, W.; Shao, S.; Lv, S.; Hu, Y. Water Quality and Microbial Community Changes in an Urban River after Micro-Nano Bubble Technology in Situ Treatment. Water 2019, 11, 66. [Google Scholar] [CrossRef] [Green Version]
- Guo, D.; Liang, J.; Chen, W.; Wang, J.; Ji, B.; Luo, S. Bacterial Community Analysis of Two Neighboring Freshwater Lakes Originating from One Lake. Pol. J. Environ. Stud. 2021, 30, 111–117. [Google Scholar] [CrossRef]
- Kang, H.; Kim, H.; Lee, B.-I.; Joung, Y.; Joh, K. Sediminibacterium goheungense sp nov. isolated from a freshwater reservoir. Int. J. Syst. Evol. Microbiol. 2014, 64, 1328–1333. [Google Scholar] [CrossRef]
- Ettamimi, S.; Carlier, J.D.; Cox, C.J.; Elamine, Y.; Hammani, K.; Ghazal, H.; Costa, M.C. A meta-taxonomic investigation of the prokaryotic diversity of water bodies impacted by acid mine drainage from the SAo Domingos mine in southern Portugal. Extremophiles 2019, 23, 821–834. [Google Scholar] [CrossRef]
- Reza, M.S.; Mizusawa, N.; Kumano, A.; Oikawa, C.; Ouchi, D.; Kobiyama, A.; Yamada, Y.; Ikeda, Y.; Ikeda, D.; Ikeo, K.; et al. Metagenomic analysis using 16S ribosomal RNA genes of a bacterial community in an urban stream, the Tama River, Tokyo. Fish. Sci. 2018, 84, 563–577. [Google Scholar] [CrossRef]
- Ruiz-Lopez, S.; Foster, L.; Boothman, C.; Cole, N.; Morris, K.; Lloyd, J.R. Identification of a Stable Hydrogen-Driven Microbiome in a Highly Radioactive Storage Facility on the Sellafield Site. Front. Microbiol. 2020, 11, 587556. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, F.; Yang, H.; Pan, M.; Du, J. Study on Microbial Community Composition and Variation based on High Throughput Sequencing under Leersia hexandra Swartz Ecological Floating Bed. Southwest China J. Agric. Sci. 2018, 31, 1903–1911. [Google Scholar]
- Wang, H.; Hu, C.; Hu, X. Effects of combined UV and chlorine disinfection on corrosion and water quality within reclaimed water distribution systems. Eng. Fail. Anal. 2014, 39, 12–20. [Google Scholar] [CrossRef]
- Singleton, D.R.; Adrion, A.C.; Aitken, M.D. Surfactant-induced bacterial community changes correlated with increased polycyclic aromatic hydrocarbon degradation in contaminated soil. Appl. Microbiol. Biotechnol. 2016, 100, 10165–10177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, F.P.; Liu, X.; Mattes, T.E.; Cupples, A.M. Nocardioides, Sediminibacterium, Aquabacterium, Variovorax, and Pseudomonas linked to carbon uptake during aerobic vinyl chloride biodegradation. Environ. Sci. Pollut. Res. 2016, 23, 19062–19070. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Song, T.; Shen, Y.; Jin, Q.; Feng, W.; Fan, L.; Cai, W. Diversity of Bacterial and Fungal Communities in Wheat Straw Compost for Agaricus bisporus Cultivation. Hortscience 2019, 54, 100–109. [Google Scholar] [CrossRef] [Green Version]
- Vita, N.; Ravachol, J.; Franche, N.; Borne, R.; Tardif, C.; Pages, S.; Fierobe, H.-P. Restoration of cellulase activity in the inactive cellulosomal protein Cel9V from Ruminiclostridium cellulolyticum. Febs Lett. 2018, 592, 190–198. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Q.; Dai, L.; Wang, Y.; Tan, F.; Chen, C.; He, M.; Maeda, T. Enrichment of waste sewage sludge for enhancing methane production from cellulose. Bioresour. Technol. 2021, 321, 124497. [Google Scholar] [CrossRef]
- Ziganshina, E.E.; Belostotskiy, D.E.; Bulynina, S.S.; Ziganshin, A.M. Effect of magnetite on anaerobic digestion of distillers grains and beet pulp: Operation of reactors and microbial community dynamics. J. Biosci. Bioeng. 2021, 131, 290–298. [Google Scholar] [CrossRef]
- Newmister, S.A.; Chan, C.H.; Escalante-Semerena, J.C.; Rayment, I. Structural Insights into the Function of the Nicotinate Mononucleotide:phenol/p-cresol Phosphoribosyltransferase (ArsAB) Enzyme from Sporomusa ovata. Biochemistry 2012, 51, 8571–8582. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.R.; Pesaro, M.; Sigler, W.; Zeyer, J. Identification of microorganisms involved in reductive dehalogenation of chlorinated ethenes in an anaerobic microbial community. Water Res. 2005, 39, 3954–3966. [Google Scholar] [CrossRef] [PubMed]
- McIlroy, S.J.; Onetto, C.A.; McIlroy, B.; Herbst, F.-A.; Dueholm, M.S.; Kirkegaard, R.H.; Fernando, E.; Karst, S.M.; Nierychlo, M.; Kristensen, J.M.; et al. Genomic and in Situ Analyses Reveal the Micropruina spp. as Abundant Fermentative Glycogen Accumulating Organisms in Enhanced Biological Phosphorus Removal Systems. Front. Microbiol. 2018, 9, 1004. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.-M.; Mo, Y.-X.; Han, L.; Nogi, Y.; Zhu, Y.-H.; Lv, J. Qipengyuania sediminis gen. nov. sp nov. a member of the family Erythrobacteraceae isolated from subterrestrial sediment. Int. J. Syst. Evol. Microbiol. 2015, 65, 3658–3665. [Google Scholar] [CrossRef]
- Amoah, K.; Huang, Q.-c.; Dong, X.-h.; Tan, B.-p.; Zhang, S.; Chi, S.-y.; Yang, Q.-h.; Liu, H.-y.; Yang, Y.-z. Paenibacillus polymyxa improves the growth, immune and antioxidant activity, intestinal health, and disease resistance in Litopenaeus vannamei challenged with Vibrio parahaemolyticus. Aquaculture 2020, 518, 734563. [Google Scholar] [CrossRef]
- Nwinyi, O.C.; Amund, O.O. Biodegradation of Selected Polycyclic Aromatic Hydrocarbons by Axenic Bacterial Species Belonging to the Genera Lysinibacillus and Paenibacillus. Iran. J. Sci. Technol. Trans. A Sci. 2017, 41, 577–587. [Google Scholar] [CrossRef]
- Shehu, D.; Alias, Z. Dechlorination of polychlorobiphenyl degradation metabolites by a recombinant glutathione S-transferase from Acidovorax sp. KKS102. Febs Open Bio 2019, 9, 408–419. [Google Scholar] [CrossRef] [Green Version]
- Fang, M.-M.; Yan, N.; Zhang, Y.-M. Biodegradation of pyridine under UV irradiation. Huan Jing Ke Xue = Huanjing Kexue 2012, 33, 488–494. [Google Scholar]
- Li, G.; Park, S.; Kang, D.-W.; Krajmalnik-Brown, R.; Rittmann, B.E. 2,4,5-Trichlorophenol Degradation Using a Novel TiO2-Coated Biofilm Carrier: Roles of Adsorption, Photocatalysis, and Biodegradation. Environ. Sci. Technol. 2011, 45, 8359–8367. [Google Scholar] [CrossRef]
- Yu, M.; Wang, J.; Tang, L.; Feng, C.; Liu, H.; Zhang, H.; Peng, B.; Chen, Z.; Xie, Q. Intimate coupling of photocatalysis and biodegradation for wastewater treatment: Mechanisms, recent advances and environmental applications. Water Res. 2020, 175, 115673. [Google Scholar] [CrossRef]












| No. | Molecular Formula | m/z | Proposed Molecular | Proposed Structure |
|---|---|---|---|---|
| M1 | C6H14O2 | 118.10 | 3-Hexyl hydroperoxide |  |
| M2 | C6H4Cl2 | 145.97 | o-Dichlorobenzene |  |
| M3 | C6H4Cl2 | 145.97 | p-Dichlorobenzene |  |
| M4 | C8H18O | 130.14 | 2-Ethylhexanol |  |
| M5 | C6H3Cl3 | 179.93 | 1,2,4-Trichlorobenzene |  |
| M6 | C10H20O | 156.15 | 2-Decenol |  |
| M7 | C10H22O | 158.17 | Tetrahydrolavandulol |  |
| M8 | C11H10O6 | 238.05 | 3,4-Bis (methoxycarbonyl) benzoic acid |  |
| M9 | C14H22O | 206.17 | 2,4-di-Butylphenol |  |
| M10 | C16H22O4 | 278.15 | 1,2-dicarboxylate -dibutyl benzene |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Z.; Jiao, C.; Liang, Y.; Du, A.; Zhang, J.; Xiong, J.; Chen, G.; Zhu, H.; Lu, L. Study on Degradation of 1,2,4-TrCB by Sugarcane Cellulose-TiO2 Carrier in an Intimate Coupling of Photocatalysis and Biodegradation System. Polymers 2022, 14, 4774. https://doi.org/10.3390/polym14214774
Zhou Z, Jiao C, Liang Y, Du A, Zhang J, Xiong J, Chen G, Zhu H, Lu L. Study on Degradation of 1,2,4-TrCB by Sugarcane Cellulose-TiO2 Carrier in an Intimate Coupling of Photocatalysis and Biodegradation System. Polymers. 2022; 14(21):4774. https://doi.org/10.3390/polym14214774
Chicago/Turabian StyleZhou, Zhenqi, Chunlin Jiao, Yinna Liang, Ang Du, Jiaming Zhang, Jianhua Xiong, Guoning Chen, Hongxiang Zhu, and Lihai Lu. 2022. "Study on Degradation of 1,2,4-TrCB by Sugarcane Cellulose-TiO2 Carrier in an Intimate Coupling of Photocatalysis and Biodegradation System" Polymers 14, no. 21: 4774. https://doi.org/10.3390/polym14214774
APA StyleZhou, Z., Jiao, C., Liang, Y., Du, A., Zhang, J., Xiong, J., Chen, G., Zhu, H., & Lu, L. (2022). Study on Degradation of 1,2,4-TrCB by Sugarcane Cellulose-TiO2 Carrier in an Intimate Coupling of Photocatalysis and Biodegradation System. Polymers, 14(21), 4774. https://doi.org/10.3390/polym14214774