Irinotecan-Loaded Polymeric Micelles as a Promising Alternative to Enhance Antitumor Efficacy in Colorectal Cancer Therapy
Abstract
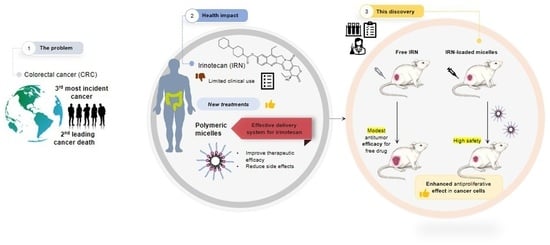
:1. Introduction
2. Materials and Methods
2.1. Preparation of Micelles
Irinotecan-Loaded Micelles
2.2. Physicochemical Characterization
2.2.1. Mean Diameter and Size Distribution
2.2.2. Zeta Potential
2.2.3. Entrapment Efficiency
2.3. Short-Term Stability Evaluation
2.4. Preparation of Freeze-Dried Micelles for IRN Encapsulation
2.5. In Vitro IRN Release Study
2.6. Hemolysis Assay
2.7. In Vivo Studies
2.7.1. Animals
2.7.2. Cell Culture
2.7.3. Antitumor Activity Evaluation
2.7.4. Histopathological Analysis
2.7.5. Toxicity Evaluation
2.8. Statistical Analysis
3. Results
3.1. Physicochemical Characterization
3.2. Short-Term Stability Evaluation
3.3. Preparation of Freeze-Dried Micelles for IRN Encapsulation
3.4. In Vitro Release Study
3.5. Hemolysis Assay
3.6. In Vivo Antitumor Activity Evaluation
3.7. Histopathological Analysis
3.8. Toxicity Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- André, T.; Louvet, C.; Maindrault-Goebel, F.; Couteau, C.; Mabro, M.; Lotz, J.; Gilles-Amar, V.; Krulik, M.; Carola, E.; Izrael, V.; et al. CPT-11 (Irinotecan) Addition to Bimonthly, High-Dose Leucovorin and Bolus and Continuous-Infusion 5-Fluorouracil (FOLFIRI) for Pretreated Metastatic Colorectal Cancer. Eur. J. Cancer 1999, 35, 1343–1347. [Google Scholar] [CrossRef]
- Zhang, H. Onivyde for the Therapy of Multiple Solid Tumors. OncoTargets Ther. 2016, 9, 3001–3007. [Google Scholar] [CrossRef] [Green Version]
- Bailly, C. Irinotecan: 25 Years of Cancer Treatment. Pharmacol. Res. 2019, 148, 104398. [Google Scholar] [CrossRef]
- Arshad, U.; Sutton, P.A.; Ashford, M.B.; Treacher, K.E.; Liptrott, N.J.; Rannard, S.P.; Goldring, C.E.; Owen, A. Critical Considerations for Targeting Colorectal Liver Metastases with Nanotechnology. WIREs Nanomed. Nanobiotechnol. 2020, 12, e1588. [Google Scholar] [CrossRef] [Green Version]
- SEZGIN, Z.; YUKSEL, N.; BAYKARA, T. Preparation and Characterization of Polymeric Micelles for Solubilization of Poorly Soluble Anticancer Drugs. Eur. J. Pharm. Biopharm. 2006, 64, 261–268. [Google Scholar] [CrossRef]
- Malaiya, A.; Jain, D.; Yadav, A.K. Nanoparticles and Pancreas Cancer. In Nano Drug Delivery Strategies for the Treatment of Cancers; Elsevier: Amsterdam, The Netherlands, 2021; pp. 145–164. [Google Scholar]
- Hussein, Y.; Youssry, M. Polymeric Micelles of Biodegradable Diblock Copolymers: Enhanced Encapsulation of Hydrophobic Drugs. Materials 2018, 11, 688. [Google Scholar] [CrossRef] [Green Version]
- Torchilin, V.P. Micellar Nanocarriers: Pharmaceutical Perspectives. Pharm. Res. 2006, 24, 1–16. [Google Scholar] [CrossRef]
- Bryant, A.A.A.; Vanden Berg-Foels, W.S.; Wen, X. Bioengineering Strategies for Designing Targeted Cancer Therapies. In Advances in Cancer Research; Elsevier: Amsterdam, The Netherlands, 2013; Volume 118, pp. 1–59. [Google Scholar] [CrossRef] [Green Version]
- Biswas, S.; Kumari, P.; Lakhani, P.M.; Ghosh, B. Recent Advances in Polymeric Micelles for Anti-Cancer Drug Delivery. Eur. J. Pharm. Sci. 2016, 83, 184–202. [Google Scholar] [CrossRef]
- Tan, C.; Wang, Y.; Fan, W. Exploring Polymeric Micelles for Improved Delivery of Anticancer Agents: Recent Developments in Preclinical Studies. Pharmaceutics 2013, 5, 201–219. [Google Scholar] [CrossRef] [Green Version]
- Elsaid, Z.; Taylor, K.M.G.; Puri, S.; Eberlein, C.A.; Al-Jamal, K.; Bai, J.; Klippstein, R.; Wang, J.T.-W.; Forbes, B.; Chana, J.; et al. Mixed Micelles of Lipoic Acid-Chitosan-Poly (Ethylene Glycol) and Distearoylphosphatidylethanolamine-Poly (Ethylene Glycol) for Tumor Delivery. Eur. J. Pharm. Sci. 2017, 101, 228–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavalcante, C.H.; Fernandes, R.S.; de Oliveira Silva, J.; Ramos Oda, C.M.; Leite, E.A.; Cassali, G.D.; Charlie-Silva, I.; Ventura Fernandes, B.H.; Miranda Ferreira, L.A.; de Barros, A.L.B. Doxorubicin-Loaded PH-Sensitive Micelles: A Promising Alternative to Enhance Antitumor Activity and Reduce Toxicity. Biomed. Pharmacother. 2021, 134, 111076. [Google Scholar] [CrossRef] [PubMed]
- Katragadda, U.; Fan, W.; Wang, Y.; Teng, Q.; Tan, C. Combined Delivery of Paclitaxel and Tanespimycin via Micellar Nanocarriers: Pharmacokinetics, Efficacy and Metabolomic Analysis. PLoS ONE 2013, 8, e58619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramasamy, T.; Choi, J.Y.; Cho, H.J.; Umadevi, S.K.; Shin, B.S.; Choi, H.-G.; Yong, C.S.; Kim, J.O. Polypeptide-Based Micelles for Delivery of Irinotecan: Physicochemical and In Vivo Characterization. Pharm. Res. 2015, 32, 1947–1956. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, Z.; Shah, A.; Siddiq, M.; Kraatz, H.-B. Polymeric Micelles as Drug Delivery Vehicles. RSC Adv. 2014, 4, 17028–17038. [Google Scholar] [CrossRef]
- Oda, C.M.R.; Silva, J.d.O.; Fernandes, R.S.; Braga, A.V.; Machado, R.d.R.; Coelho, M.d.M.; Cassali, G.D.; Reis, D.C.; de Barros, A.L.B.; Leite, E.A. Encapsulating Paclitaxel in Polymeric Nanomicelles Increases Antitumor Activity and Prevents Peripheral Neuropathy. Biomed. Pharmacother. 2020, 132, 110864. [Google Scholar] [CrossRef]
- Li, C.; Xu, J.; Gan, Y.; Liang, X.-J. Innovative Irinotecan-Loaded Nanomicelles Will Enter Phase I Clinical Trial in 2021. Innovation 2020, 1, 100057. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, R.; Lu, X.; Lu, W.; Zhang, C.; Liang, W. Pegylated Phospholipids-Based Self-Assembly with Water-Soluble Drugs. Pharm. Res. 2010, 27, 361–370. [Google Scholar] [CrossRef]
- Din, F.; Kim, D.W.; Choi, J.Y.; Thapa, R.K.; Mustapha, O.; Kim, D.S.; Oh, Y.-K.; Ku, S.K.; Youn, Y.S.; Oh, K.T.; et al. Irinotecan-Loaded Double-Reversible Thermogel with Improved Antitumor Efficacy without Initial Burst Effect and Toxicity for Intramuscular Administration. Acta Biomater. 2017, 54, 239–248. [Google Scholar] [CrossRef]
- Yan, J.; Wang, F.; Chen, J.; Liu, T.; Zhang, T. Preparation and Characterization of Irinotecan Loaded Cross-Linked Bovine Serum Albumin Beads for Liver Cancer Chemoembolization Therapy. Int. J. Polym. Sci. 2016, 2016, 9651486. [Google Scholar] [CrossRef] [Green Version]
- Lages, E.B.; Fernandes, R.S.; Andrade, M.M.S.; Paiyabhroma, N.; de Oliveira, R.B.; Fernandes, C.; Cassali, G.D.; Sicard, P.; Richard, S.; Branco de Barros, A.L.; et al. PH-Sensitive Doxorubicin-Tocopherol Succinate Prodrug Encapsulated in Docosahexaenoic Acid-Based Nanostructured Lipid Carriers: An Effective Strategy to Improve Pharmacokinetics and Reduce Toxic Effects. Biomed. Pharmacother. 2021, 144, 112373. [Google Scholar] [CrossRef] [PubMed]
- Boratto, F.A.; Franco, M.S.; Barros, A.L.B.; Cassali, G.D.; Malachias, A.; Ferreira, L.A.M.; Leite, E.A. Alpha-Tocopheryl Succinate Improves Encapsulation, PH-Sensitivity, Antitumor Activity and Reduces Toxicity of Doxorubicin-Loaded Liposomes. Eur. J. Pharm. Sci. 2019, 144, 105205. [Google Scholar] [CrossRef] [PubMed]
- Tardi, P.G.; Gallagher, R.C.; Johnstone, S.; Harasym, N.; Webb, M.; Bally, M.B.; Mayer, L.D. Coencapsulation of Irinotecan and Floxuridine into Low Cholesterol-Containing Liposomes That Coordinate Drug Release in Vivo. Biochim. Biophys. Acta Biomembr. 2007, 1768, 678–687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nunes, S.S.; Miranda, S.E.M.; de Oliveira Silva, J.; Fernandes, R.S.; de Alcântara Lemos, J.; de Aguiar Ferreira, C.; Townsend, D.M.; Cassali, G.D.; Oliveira, M.C.; Branco de Barros, A.L. PH-Responsive and Folate-Coated Liposomes Encapsulating Irinotecan as an Alternative to Improve Efficacy of Colorectal Cancer Treatment. Biomed. Pharmacother. 2021, 144, 112317. [Google Scholar] [CrossRef] [PubMed]
- Poujol, S.; Pinguet, F.; Malosse, F.; Astre, C.; Ychou, M.; Culine, S.; Bressolle, F. Sensitive HPLC-Fluorescence Method for Irinotecan and Four Major Metabolites in Human Plasma and Saliva: Application to Pharmacokinetic Studies. Clin. Chem. 2003, 49, 1900–1908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miranda, S.E.M.; Lemos, J.d.A.; Fernandes, R.S.; Silva, J.d.O.; Ottoni, F.M.; Townsend, D.M.; Rubello, D.; Alves, R.J.; Cassali, G.D.; Ferreira, L.A.M.; et al. Enhanced Antitumor Efficacy of Lapachol-Loaded Nanoemulsion in Breast Cancer Tumor Model. Biomed. Pharmacother. 2021, 133, 110936. [Google Scholar] [CrossRef] [PubMed]
- Heske, C.; Mendoza, A.; Yeung, C.; Proia, D.A.; Neckers, L.; Helman, L.J. Activity of Hsp90-Inhibitor Drug Conjugate (HDC) STA-12-8666 in Preclinical Models of Pediatric Sarcoma. J. Clin. Oncol. 2015, 33, 10025. [Google Scholar] [CrossRef]
- Silva, J.d.O.; Miranda, S.E.M.; Leite, E.A.; de Paula Sabino, A.; Borges, K.B.G.; Cardoso, V.N.; Cassali, G.D.; Guimarães, A.G.; Oliveira, M.C.; de Barros, A.L.B. Toxicological Study of a New Doxorubicin-Loaded PH-Sensitive Liposome: A Preclinical Approach. Toxicol. Appl. Pharmacol. 2018, 352, 162–169. [Google Scholar] [CrossRef]
- Sato, T.; Sakai, H.; Sou, K.; Buchner, R.; Tsuchida, E. Poly (Ethylene Glycol)-Conjugated Phospholipids in Aqueous Micellar Solutions: Hydration, Static Structure, and Interparticle Interactions. J. Phys. Chem. B 2007, 111, 1393–1401. [Google Scholar] [CrossRef]
- Xu, M.; Yao, C.; Zhang, W.; Gao, S.; Zou, H.; Gao, J. Anti-Cancer Activity Based on the High Docetaxel Loaded Poly (2-Oxazoline)s Micelles. Int. J. Nanomed. 2021, 16, 2735–2749. [Google Scholar] [CrossRef]
- Cabral, H.; Miyata, K.; Osada, K.; Kataoka, K. Block Copolymer Micelles in Nanomedicine Applications. Chem. Rev. 2018, 118, 6844–6892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabral, H.; Matsumoto, Y.; Mizuno, K.; Chen, Q.; Murakami, M.; Kimura, M.; Terada, Y.; Kano, M.R.; Miyazono, K.; Uesaka, M.; et al. Accumulation of Sub-100 Nm Polymeric Micelles in Poorly Permeable Tumours Depends on Size. Nat. Nanotechnol. 2011, 6, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Fan, Q.; Wang, J.; Zhang, B.; Hao, T.; Chen, Q.; Li, L.; Chen, L.; Cui, H.; Li, Z. PEGylated Phospholipid Micelles Containing D-α-Tocopheryl Succinate as Multifunctional Nanocarriers for Enhancing the Antitumor Efficacy of Doxorubicin. Int. J. Pharm. 2021, 607, 120979. [Google Scholar] [CrossRef] [PubMed]
- Garbuzenko, O.; Zalipsky, S.; Qazen, M.; Barenholz, Y. Electrostatics of PEGylated Micelles and Liposomes Containing Charged and Neutral Lipopolymers. Langmuir 2005, 21, 2560–2568. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Moulder, K.R.; Cohen, M.S.; Cai, S.; Forrest, L.M. Cabozantinib Loaded DSPE-PEG2000 Micelles as Delivery System: Formulation, Characterization and Cytotoxicity Evaluation. BAOJ Pharm. Sci. 2015, 1, 25688382. [Google Scholar]
- Pei, X.; Luo, F.; Zhang, J.; Chen, W.; Jiang, C.; Liu, J. Dehydroascorbic Acids-Modified Polymer Micelles Target Cancer Cells to Enhance Anti-Tumor Efficacy of Paclitaxel. Sci. Rep. 2017, 7, 975. [Google Scholar] [CrossRef] [Green Version]
- Dobrovolskaia, M.A.; McNeil, S.E. Handbook of Immunological Properties of Engineered Nanomaterials, 2nd ed.; Dobrovolskaia, M.A., McNeil, S.E., Eds.; World Scientific Publishing Company: Hackensack, NJ, USA, 2016; ISBN 9789814699167. [Google Scholar]
- Lu, Y.-J.; Lin, P.-Y.; Huang, P.-H.; Kuo, C.-Y.; Shalumon, K.T.; Chen, M.-Y.; Chen, J.-P. Magnetic Graphene Oxide for Dual Targeted Delivery of Doxorubicin and Photothermal Therapy. Nanomaterials 2018, 8, 193. [Google Scholar] [CrossRef] [Green Version]
- Li, K.-C.; Chu, H.-C.; Lin, Y.; Tuan, H.-Y.; Hu, Y.-C. PEGylated Copper Nanowires as a Novel Photothermal Therapy Agent. ACS Appl. Mater. Interfaces 2016, 8, 12082–12090. [Google Scholar] [CrossRef]
- Oda, C.M.R.; Fernandes, R.S.; de Araújo Lopes, S.C.; de Oliveira, M.C.; Cardoso, V.N.; Santos, D.M.; de Castro Pimenta, A.M.; Malachias, A.; Paniago, R.; Townsend, D.M.; et al. Synthesis, Characterization and Radiolabeling of Polymeric Nano-Micelles as a Platform for Tumor Delivering. Biomed. Pharmacother. 2017, 89, 268–275. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Cai, T.; Huang, Y.; Xia, X.; Cole, S.; Cai, Y. A Review of the Structure, Preparation, and Application of NLCs, PNPs, and PLNs. Nanomaterials 2017, 7, 122. [Google Scholar] [CrossRef]
- Takayama, R.; Inoue, Y.; Murata, I.; Kanamoto, I. Characterization of Nanoparticles Using DSPE-PEG2000 and Soluplus. Colloids Interfaces 2020, 4, 28. [Google Scholar] [CrossRef]
- Dabholkar, R.D.; Sawant, R.M.; Mongayt, D.A.; Devarajan, P.V.; Torchilin, V.P. Polyethylene Glycol–Phosphatidylethanolamine Conjugate (PEG–PE)-Based Mixed Micelles: Some Properties, Loading with Paclitaxel, and Modulation of P-Glycoprotein-Mediated Efflux. Int. J. Pharm. 2006, 315, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Lukyanov, A.N.; Torchilin, V.P. Micelles from Lipid Derivatives of Water-Soluble Polymers as Delivery Systems for Poorly Soluble Drugs. Adv. Drug Deliv. Rev. 2004, 56, 1273–1289. [Google Scholar] [CrossRef] [PubMed]
- Musacchio, T.; Laquintana, V.; Latrofa, A.; Trapani, G.; Torchilin, V.P. PEG-PE Micelles Loaded with Paclitaxel and Surface-Modified by a PBR-Ligand: Synergistic Anticancer Effect. Mol. Pharm. 2009, 6, 468–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Croy, S.; Kwon, G. Polymeric Micelles for Drug Delivery. Curr. Pharm. Des. 2006, 12, 4669–4684. [Google Scholar] [CrossRef]
- Alexis, F.; Pridgen, E.; Molnar, L.K.; Farokhzad, O.C. Factors Affecting the Clearance and Biodistribution of Polymeric Nanoparticles. Mol. Pharm. 2008, 5, 505–515. [Google Scholar] [CrossRef] [Green Version]
- Qian, J.; Gao, X. Triblock Copolymer-Encapsulated Nanoparticles with Outstanding Colloidal Stability for SiRNA Delivery. ACS Appl. Mater. Interfaces 2013, 5, 2845–2852. [Google Scholar] [CrossRef]
- Yao, Y.; Zhou, Y.; Liu, L.; Xu, Y.; Chen, Q.; Wang, Y.; Wu, S.; Deng, Y.; Zhang, J.; Shao, A. Nanoparticle-Based Drug Delivery in Cancer Therapy and Its Role in Overcoming Drug Resistance. Front. Mol. Biosci. 2020, 7, 193. [Google Scholar] [CrossRef]
- Chou, W.-H.; Galaz, A.; Jara, M.O.; Gamboa, A.; Morales, J.O. Drug-Loaded Lipid-Core Micelles in Mucoadhesive Films as a Novel Dosage Form for Buccal Administration of Poorly Water-Soluble and Biological Drugs. Pharmaceutics 2020, 12, 1168. [Google Scholar] [CrossRef]
- Wang, J.; Xing, X.; Fang, X.; Zhou, C.; Huang, F.; Wu, Z.; Lou, J.; Liang, W. Cationic Amphiphilic Drugs Self-Assemble to the Core–Shell Interface of PEGylated Phospholipid Micelles and Stabilize Micellar Structure. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2013, 371, 20120309. [Google Scholar] [CrossRef]
- Hari, S.K.; Gauba, A.; Shrivastava, N.; Tripathi, R.M.; Jain, S.K.; Pandey, A.K. Polymeric Micelles and Cancer Therapy: An Ingenious Multimodal Tumor-Targeted Drug Delivery System. Drug Deliv. Transl. Res. 2022, in press. [Google Scholar] [CrossRef]
- Wen, S.-N.; Chu, C.-H.; Wang, Y.-C.; Huang, H.-Y.; Wang, Y.-J.; Lin, J.-Y.; Lu, H.-T.; Wang, S.-J.; Yang, C.-S. Polymer-Stabilized Micelles Reduce the Drug Rapid Clearance In Vivo. J. Nanomater. 2018, 2018, 5818592. [Google Scholar] [CrossRef] [Green Version]
- Ojha, T.; Hu, Q.; Colombo, C.; Wit, J.; Geijn, M.; Steenbergen, M.J.; Bagheri, M.; Königs-Werner, H.; Buhl, E.M.; Bansal, R.; et al. Lyophilization Stabilizes Clinical-stage Core-crosslinked Polymeric Micelles to Overcome Cold Chain Supply Challenges. Biotechnol. J. 2021, 16, 2000212. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, M. Polymeric Micelles as Drug Carriers: Their Lights and Shadows. J. Drug Target. 2014, 22, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Sawant, R.R.; Torchilin, V.P. Polymeric Micelles: Polyethylene Glycol-Phosphatidylethanolamine (PEG-PE)-Based Micelles as an Example. In Cancer Nanotechnology; Humana Press: Totowa, NJ, USA, 2010; pp. 131–149. [Google Scholar]
- Torchilin, V. Lipid-Core Micelles for Targeted Drug Delivery. Curr. Drug Deliv. 2005, 2, 319–327. [Google Scholar] [CrossRef] [PubMed]
- D’souza, A.A.; Shegokar, R. Polyethylene Glycol (PEG): A Versatile Polymer for Pharmaceutical Applications. Expert Opin. Drug Deliv. 2016, 13, 1257–1275. [Google Scholar] [CrossRef]
- Castillo, T.D.C.; Carmona, J.S.C.; Velasco, A.L.; Contreras, E.A.Z. PH-Responsive Polymer Micelles for Methotrexate Delivery at Tumor Microenvironments. e-Polymers 2020, 20, 624–635. [Google Scholar] [CrossRef]
- Guo, S.; Shi, Y.; Liang, Y.; Liu, L.; Sun, K.; Li, Y. Relationship and Improvement Strategies between Drug Nanocarrier Characteristics and Hemocompatibility: What Can We Learn from the Literature. Asian J. Pharm. Sci. 2021, 16, 551–576. [Google Scholar] [CrossRef]
- Zhao, B.; Wang, X.-Q.; Wang, X.-Y.; Zhang, H.; Dai, W.-B.; Wang, J.; Zhong, Z.-L.; Wu, H.-N.; Zhang, Q. Nanotoxicity Comparison of Four Amphiphilic Polymeric Micelles with Similar Hydrophilic or Hydrophobic Structure. Part. Fibre Toxicol. 2013, 10, 47. [Google Scholar] [CrossRef]







| Formulation | Mean Diameter (nm) a | Size Distribution b | Zeta Potential (mV) | EE (%) |
|---|---|---|---|---|
| PM DSPE-PEG | 12.4 ± 0.3 | ~90% < 20 nm | −2.4 ± 0.9 | |
| PM DSPE-PEG IRN | 12.6 ± 1.2 | ~90% < 20 nm | −2.6 ± 1.7 | 88.7 ± 4.4 |
| IRN Theoretical Concentration | Mean Diameter (nm) | Zeta Potential (mV) | Size Distribution (<20 nm) | %EE | IRN-Loading (µg/mL) |
|---|---|---|---|---|---|
| 1 mg/mL | 12.6 ± 1.2 | −2.6 ± 1.7 | 97.5 ± 0.1 | 88.7 ± 4.4 | 743 ± 99 |
| 2 mg/mL | 12.5 ± 2.4 | 0.6 ± 4.7 | 97.8 ± 2.8 | 80.2 ± 3.2 | 1258 ± 75 |
| 3 mg/mL | 12.3 ± 1.1 | −0.5 ± 0.4 | 99.3 ± 0.4 | 68.5 ± 10.5 | 2055 ± 382 |
| Group | RTV (Mean ± SD) | IR (%) |
|---|---|---|
| Control | 18.7 ± 9.8 | |
| Free IRN | 5.9 ± 2.0 * | 68.7 |
| PM DSPE-PEG IRN | 2.1 ± 0.3 *,# | 88.9 |
| Parameters | Control | Free IRN | PM DSPE-PEG IRN |
|---|---|---|---|
| RDW (cell/mm3 × 103) | 5.3 ± 0.8 | 4.3 ± 1.0 | 5.7 ± 0.5 |
| LYM (cell/mm3 × 103) | 1.5 ± 0.4 | 0.9 ± 0.2 | 1.2 ± 0.4 |
| Nph (cell/mm3 × 103) | 2.7 ± 0.6 | 2.6 ± 0.8 | 2.5 ± 0.4 |
| RBC (cell/mm3 × 106) | 6.3 ± 0.2 | 5.9 ± 0.4 | 5.7 ± 0.5 |
| HGB (g/L) | 11.7 ± 0.6 | 11.1 ± 0.9 | 10.3 ± 1.4 |
| HTC (%) | 30.6 ± 1.1 | 30.8 ± 1.9 | 28.6 ± 2.8 |
| RDW (%) | 14.7 ± 0.6 | 16.0 ± 1.1 | 14.7 ± 0.7 |
| PLT (cell/mm3 × 103) | 380.3 ± 75.6 | 323. 7 ± 41.2 | 314.5 ± 98.0 |
| ALT (U/L) | 22.7 ± 5.5 | 23.3 ± 2.5 | 23.1 ± 3.6 |
| AST (U/L) | 146.4 ± 45.4 | 151. 8 ± 27.6 | 151.3 ± 34.5 |
| Urea (mg/dL) | 70.1 ± 7.2 | 83.2 ± 14.0 | 54.3 ± 9.6 |
| Creatinine (mg/dL) | 0.42 ± 0.10 | 0.39 ± 0.03 | 0.32 ± 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campos, F.L.; de Alcântara Lemos, J.; Oda, C.M.R.; de Oliveira Silva, J.; Fernandes, R.S.; Miranda, S.E.M.; Cavalcante, C.H.; Cassali, G.D.; Townsend, D.M.; Leite, E.A.; et al. Irinotecan-Loaded Polymeric Micelles as a Promising Alternative to Enhance Antitumor Efficacy in Colorectal Cancer Therapy. Polymers 2022, 14, 4905. https://doi.org/10.3390/polym14224905
Campos FL, de Alcântara Lemos J, Oda CMR, de Oliveira Silva J, Fernandes RS, Miranda SEM, Cavalcante CH, Cassali GD, Townsend DM, Leite EA, et al. Irinotecan-Loaded Polymeric Micelles as a Promising Alternative to Enhance Antitumor Efficacy in Colorectal Cancer Therapy. Polymers. 2022; 14(22):4905. https://doi.org/10.3390/polym14224905
Chicago/Turabian StyleCampos, Fernanda Lapa, Janaina de Alcântara Lemos, Caroline Mari Ramos Oda, Juliana de Oliveira Silva, Renata Salgado Fernandes, Sued Eustaquio Mendes Miranda, Carolina Henriques Cavalcante, Geovanni Dantas Cassali, Danyelle M. Townsend, Elaine Amaral Leite, and et al. 2022. "Irinotecan-Loaded Polymeric Micelles as a Promising Alternative to Enhance Antitumor Efficacy in Colorectal Cancer Therapy" Polymers 14, no. 22: 4905. https://doi.org/10.3390/polym14224905
APA StyleCampos, F. L., de Alcântara Lemos, J., Oda, C. M. R., de Oliveira Silva, J., Fernandes, R. S., Miranda, S. E. M., Cavalcante, C. H., Cassali, G. D., Townsend, D. M., Leite, E. A., & de Barros, A. L. B. (2022). Irinotecan-Loaded Polymeric Micelles as a Promising Alternative to Enhance Antitumor Efficacy in Colorectal Cancer Therapy. Polymers, 14(22), 4905. https://doi.org/10.3390/polym14224905