Ferrocene-Based Terpolyamides and Their PDMS-Containing Block Copolymers: Synthesis and Physical Properties
Abstract
:1. Introduction
2. Experimental Materials
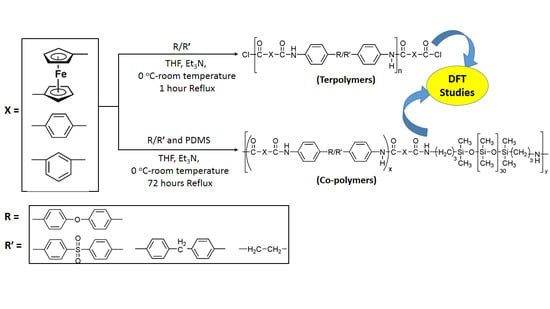
2.1. Synthesis
2.2. Physical Characterization
3. Results and Discussion
3.1. Synthesis and Characterization
3.2. Solubility
3.3. Molecular Weight Determination
3.4. Wide Angle X-ray Diffraction
3.5. Surface Morphology
3.6. Thermal Properties
3.7. Water Absorption Studies
3.8. DFT Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- El-Ghoul, Y.; Alminderej, F.M.; Alsubaie, F.M.; Alrasheed, R.; Almousa, N.H. Recent Advances in Functional Polymer Materials for Energy, Water, and Biomedical Applications: A Review. Polymers 2021, 13, 4327. [Google Scholar] [CrossRef] [PubMed]
- Gore, P.; Kandasubramanian, B. Functionalized Aramid Fibers and Composites for Protective Applications: A Review. Ind. Eng. Chem. Res. 2018, 57, 16537–16563. [Google Scholar] [CrossRef]
- Reglero Ruiz, J.A.; Trigo-López, M.; García, F.C.; García, J.M.J.P. Functional aromatic polyamides. Polymers 2017, 9, 414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, B.; Jia, L.; Tian, M.; Ning, N.; Zhang, L.; Wang, W. Surface and interface modification of aramid fiber and its reinforcement for polymer composites: A review. Eur. Polym. J. 2021, 147, 110352. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, Z.; Yuan, J.; Wu, L.; Li, P.; Zhao, X.; Men, X. Enhanced mechanical and tribological properties of Kevlar/PTFE-phenolic composites by improving interfacial properties by aramid nanofibers. Polym. Compos. 2020, 41, 4192–4201. [Google Scholar] [CrossRef]
- Li, M.; Cheng, K.; Wang, C.; Lu, S. Functionalize Aramid Fibers with Polydopamine to Possess UV Resistance. J. Inorg. Organomet. Polym. Mater. 2021, 31, 2791–2805. [Google Scholar] [CrossRef]
- Yadav, R.; Naebe, M.; Wang, X.; Kandasubramanian, B. Aramid Polycarbonate Resin Film Engineered Composite for Ballistic Protection: Engineered Layered Materials. In Recent Advances in Layered Materials and Structures; Springer: Singapore, 2021; pp. 49–66. [Google Scholar] [CrossRef]
- Qi, X.; Wang, W.; Zhang, J.; Yan, C.; Zhu, Y.; Zhang, F. Aqueously soluble semi-aromatic copolyamides with dual-functional groups as sizing agent. Polym. Sci. 2019, 136, 47132. [Google Scholar] [CrossRef]
- Endo, T.; Higashihara, T. Direct Synthesis of Thermally Stable Semiaromatic Polyamides by Bulk Polymerization Using Aromatic Diamines and Aliphatic Dicarboxylic Acids. ACS Omega 2022, 7, 8753–8758. [Google Scholar] [CrossRef]
- Farahbakhsh, J.; Vatanpour, V.; Khoshnam, M.; Zargar, M. Recent advancements in the application of new monomers and membrane modification techniques for the fabrication of thin film composite membranes: A review. React. Funct. Polym. 2021, 166, 105015. [Google Scholar] [CrossRef]
- Cai, J.; Liu, Z.; Cao, B.; Guan, X.; Liu, S.; Zhao, J. Simultaneous Improvement of the Processability and Mechanical Properties of Polyamide-6 by Chain Extension in Extrusion. Ind. Eng. Chem. Res. 2020, 59, 14334–14343. [Google Scholar] [CrossRef]
- Shoji, Y.; Mizoguchi, K.; Ueda, M. Synthesis of Aramids by Polycondensation of Aromatic Dicarboxylic Acids with Aromatic Diamines Containing Ether Linkages. Polym. J. 2008, 40, 680–681. [Google Scholar] [CrossRef] [Green Version]
- Higashihara, T.; Zhang, C.; Tsukuda, A.; Ochi, T.; Ueda, M. Direct synthesis and melt-drawing property of aramids by bulk polycondensation of isophthalic acid with m-phenylenediamine and 3, 4′-oxydianiline. J. Appl. Polym. Sci. 2012, 124, 4398–4402. [Google Scholar] [CrossRef]
- Lin, G.; Lin, X.; Qian, H.; Lin, Z. Processability of polysulfone improved by adding polyamide 6 and polysulfone-polyamide 6 block copolymer. J. Appl. Polym. Sci. 2014, 131, 41139. [Google Scholar] [CrossRef]
- Wang, Y.; Astruc, D.; Abd-El-Aziz, A.S. Metallopolymers for advanced sustainable applications. Chem. Soc. Rev. 2019, 48, 558–636. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, L.; Liu, F.; Astruc, D.; Gu, H. Supramolecular redox-responsive ferrocene hydrogels and microgels. Coord. Chem. Rev. 2020, 419, 213406. [Google Scholar] [CrossRef]
- Khan, T.; Akhter, Z.; Gul, A.; Bhatti, A.S.; Rehman, A. Facile Synthesis of Ferrocene-Based Polyamides and Their Organic Analogues Terpolyamides: Influence of Aliphatic and Aromatic Sequences on Physico-Chemical Characteristics. J. Inorg. Organomet. Polym. Mater. 2022, 32, 2928–2939. [Google Scholar] [CrossRef]
- Khan, M.S.U.; Janjua, N.K.; Sabahat, S.; Akhter, Z.; Ullah, M. Study on thermal, spectroscopic and electrochemical behavior of some ferrocene-containing organometallic polyesteramides and their siloxane-based block copolymers. J. Polym. Res. 2016, 23, 112. [Google Scholar] [CrossRef]
- Dzhardimalieva, G.I.; Yadav, B.C.; Singh, S.; Uflyand, I.E. Self-healing and shape memory metallopolymers: State-of-the-art and future perspectives. Dalton Trans. 2020, 49, 3042–3087. [Google Scholar] [CrossRef]
- Zhu, W.; Zhang, C.; Chen, Y.; Deng, Q. Synthesis of Magnetic Ferrocene-Containing Polymer with Photothermal Effects for Rapid Degradation of Methylene Blue. Polymers 2021, 13, 558. [Google Scholar] [CrossRef]
- Zhao, X.; Qi, Y.; Li, J.; Ma, Q. Porous Organic Polymers Derived from Ferrocene and Tetrahedral Silicon-Centered Monomers for Carbon Dioxide Sorption. Polymers 2022, 14, 370. [Google Scholar] [CrossRef]
- Wang, L.; Yu, H. Synthesis, Properties and Applications of Ferrocene-Based Polymers. In Synthesis, Properties and Applications of Ferrocene-Based Derivatives, Polymers and Hydrogels; Springer: Singapore, 2018; pp. 39–90. [Google Scholar] [CrossRef]
- Pietschnig, R. Polymers with pendant ferrocenes. Chem. Soc. Rev. 2016, 45, 5216–5231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, M.; Nothling, M.D.; Zhang, S.; Fu, Q.; Qiao, G.G. Thin film composite membranes for postcombustion carbon capture: Polymers and beyond. Prog. Polym. Sci. 2022, 126, 101504. [Google Scholar] [CrossRef]
- Miranda, I.; Souza, A.; Sousa, P.; Ribeiro, J.; Castanheira, E.M.S.; Lima, R.; Minas, G. Properties and Applications of PDMS for Biomedical Engineering: A Review. J. Funct. Biomater. 2022, 13, 2. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Liu, J. Synthesis and gas transport properties of polyamide membranes containing PDMS groups. RSC Adv. 2019, 9, 9737–9744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.-Y.; Huang, D.-N.; Ren, K.-F.; Ji, J. Inorganic-polymer composite coatings for biomedical devices. Smart Mater. Med. 2021, 2, 1–14. [Google Scholar] [CrossRef]
- Shakeri, A.; Khan, S.; Didar, T.F. Conventional and emerging strategies for the fabrication and functionalization of PDMS-based microfluidic devices. Lab A Chip 2021, 21, 3053–3075. [Google Scholar] [CrossRef]
- Vogel, M.; Rausch, M.; Rosenberg, H. Derivatives of Ferrocene. III. The Preparation of Some Acylferrocenes and Alkylferrocenes1. J. Org. Chem. 1957, 22, 1016–1018. [Google Scholar] [CrossRef]
- Sonoda, I.; Moritani, I. Reactions of ferrocenylcarbene iv*. the synthesis of [3]-ferrocenophan-2-one tosylhydrazone and the thermal decomposition of its sodium salt. J. Organomet. Chem. 1971, 26, 133–140. [Google Scholar] [CrossRef]
- Diwate, A.V.; Tamboli, A.M.; Ghodke, S.D.; Tamboli, A.B.; Tamboli, M.S.; Maldar, N.N. New polyamides based on diacid with decanediamide and methylene groups and aromatic diamines. J. Appl. Polym. Sci. 2022, 139, 52221. [Google Scholar] [CrossRef]
- Khan, M.S.U.; Akhter, Z.; Iqbal, N.; Siddiq, M. Synthesis, characterization and morphological studies of some novel siloxane-based block copolymeric materials containing organometallic as well as organic polyesteramides. J. Organomet. Chem. 2013, 745-746, 312–328. [Google Scholar] [CrossRef]
- Bains, W.; Petkowski, J.; Zhan, Z.; Seager, S. Evaluating Alternatives to Water as Solvents for Life: The Example of Sulfuric Acid. Life 2021, 11, 400. [Google Scholar] [CrossRef]
- Shaheen, M.E.; Ghazy, A.R.; Kenawy, E.-R.; El-Mekawy, F. Application of laser light scattering to the determination of molecular weight, second virial coefficient, and radius of gyration of chitosan. Polymer 2018, 158, 18–24. [Google Scholar] [CrossRef]
- Guo, M.; Huang, Y.; Cao, J.; Feng, S. Synthesis, properties, and degradation behaviors of novel polysulfone-polysiloxane multi-block copolymers. Polymer 2021, 212, 123134. [Google Scholar] [CrossRef]
- Ditte, K.; Perez, J.; Chae, S.; Hambsch, M.; Al-Hussein, M.; Komber, H.; Formanek, P.; Mannsfeld, S.C.B.; Fery, A.; Kiriy, A.; et al. Ultrasoft and High-Mobility Block Copolymers for Skin-Compatible Electronics. Adv. Mater. 2021, 33, e2005416. [Google Scholar] [CrossRef]
- Erceg, T.; Tanasić, J.; Banjanin, B.; Baloš, S.; Cvetinov, M.; Cakić, S.; Ristić, I. Surface, structural, and thermal properties of polydimethylsiloxane-based polyurethanes and their blends with thermoplastic polyurethane elastomer. Polym. Bull. 2022, 79, 10909–10929. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, Y.; Huang, S.; Wen, S.; Bao, X.; Cai, M.; Li, J. Impact of backbone linkage positions on the molecular aggregation behavior of polymer photovoltaic materials. Phys. Chem. Chem. Phys. 2022, 24, 17462–17470. [Google Scholar] [CrossRef]
- Khan, M.S.U.; Akhter, Z.; Naz, T.; Bhatti, A.S.; Siddiqi, H.M.; Siddiq, M.; Khan, A. Study on the preparation and properties of novel block copolymeric materials based on structurally modified organometallic as well as organic polyamides and polydimethylsiloxane. Polym. Int. 2013, 62, 319–334. [Google Scholar] [CrossRef]
- Khadivi, P.; Salami-Kalajahi, M.; Roghani-Mamaqani, H.; Sofla, R.L.M. Polydimethylsiloxane-based Polyurethane/cellulose Nanocrystal Nanocomposites: From Structural Properties Toward Cytotoxicity. Silicon 2022, 14, 1695–1703. [Google Scholar] [CrossRef]
- Ji, S.; Gui, H.; Guan, G.; Zhou, M.; Guo, Q.; Tan, M.Y. A multi-functional coating based on acrylic copolymer modified with PDMS through copolymerization. Prog. Org. Coatings 2021, 156, 106254. [Google Scholar] [CrossRef]
- Usman, M.; Yu, H.; Wang, L.; Titinchi, S.; Khan, A.; Naveed, K.-U.; Nazir, A.; Elshaarani, T.; Fahad, S.; Amin, B.U. Synthesis of poly(2-(methacryloyloxy) ethyl ferrocene carboxylate-co-glycidyl methacrylic acid)s and their anti-migration and burning rate catalytic properties. J. Therm. Anal. 2021, 146, 2445–2462. [Google Scholar] [CrossRef]
- Reyes, L.Q.; Issazadeh, S.; Zhang, J.; Dao, B.; Varley, R.J. Synthesis of Tri-Aryl Methane Epoxy Resin Isomers and Their Cure with Aromatic Amines. Macromol. Mater. Eng. 2020, 305, 1900546. [Google Scholar] [CrossRef]
- Fatima, K.; Gul, A.; Akhter, Z.; Rahman, R. Ferrocene Based Azo Chromophore Functionalized Polyesters and Its Organic Analogue: Synthesis, Structural Elucidation, Thermal Behavior and IV Characterization. J. Inorg. Organomet. Polym. Mater. 2017, 27, 474–480. [Google Scholar] [CrossRef]
- Balasubramanian, R.; Rigana, M.I.F.; Thirukumaran, P.; Atchudan, R.; Sarojadevi, M.; Lee, J. Synthesis and properties of polytriazoleimide containing anthracene, pyridine and 1, 2, 3-triazole groups and their nanocomposites with titanium dioxide. Polym. Eng. Sci. 2019, 59, 129–138. [Google Scholar] [CrossRef]
- Battig, A.; Markwart, J.C.; Wurm, F.R.; Schartel, B. Sulfur’s role in the flame retardancy of thio-ether–linked hyperbranched polyphosphoesters in epoxy resins. Eur. Polym. J. 2020, 122, 109390. [Google Scholar] [CrossRef]








| Solvents | F1 | F2 | F3 | T1 | T2 | T3 | I1 | I2 | I3 | PF1 | PF2 | PF3 | PT1 | PT2 | PT3 | PI1 | PI2 | PI3 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DMSO | ++ | ++ | ++ | -- | -- | ++ | -- | ++ | ++ | ++ | +- | +- | +- | -- | -- | +- | +- | +- |
| DMF | +- | +- | +- | -- | -- | -- | -- | -- | -- | +- | -- | +-- | -- | -- | -- | -- | -- | -- |
| THF | +- | ++ | +- | -- | -- | -- | -- | -- | -- | +- | -- | +- | -- | -- | -- | -- | -- | -- |
| m-Cresol | -- | +- | +- | -- | -- | -- | -- | -- | -- | +- | -- | +- | -- | -- | -- | -- | -- | -- |
| NMP | ++ | ++ | ++ | -- | -- | -- | -- | ++ | +- | ++ | -- | ++ | -- | -- | -- | -- | ++ | +- |
| CHCl3 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| CH2Cl2 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Acetone | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| CH3CN | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| CH3OH | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| C2H5OH | ++ | -- | ++ | -- | -- | -- | -- | -- | -- | ++ | -- | -- | -- | -- | -- | -- | -- | -- |
| (C2H5)2O | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| EtOAc | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| n-C6H14 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Toluene | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| H2SO4 | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
| Sample Codes | Tg (°C) | T10 (°C) | Tmax (°C) | Tf (°C) | CR | LOI |
|---|---|---|---|---|---|---|
| F1 | 270 | 280 | 580 | 830 | 31 | 29.9 |
| F2 | 220 | 230 | 510 | 710 | 27 | 28.3 |
| F3 | 210 | 220 | 440 | 765 | 15 | 23.5 |
| T1 | 210 | 220 | 580 | 780 | 31 | 29.9 |
| T2 | 190 | 210 | 520 | 801 | 29 | 29.1 |
| T3 | 185 | 193 | 435 | 685 | 10 | 21.5 |
| I1 | 186 | 192 | 560 | 804 | 01 | 17.9 |
| I2 | 185 | 200 | 5100 | 848 | 02 | 18.3 |
| I3 | 175 | 190 | 500 | 854 | 01 | 17.9 |
| PF1 | 220 | 240 | 600 | 834 | 40 | 33.5 |
| PF2 | 200 | 280 | 440 | 581 | 30 | 29.5 |
| PF3 | 190 | 210 | 400 | 550 | 25 | 27.5 |
| PT1 | 200 | 230 | 530 | 826 | 38 | 32.7 |
| PT2 | 180 | 200 | 550 | 762 | 35 | 31.5 |
| PT3 | 185 | 192 | 480 | 811 | 28 | 28.7 |
| PI1 | 190 | 198 | 510 | 846 | 30 | 29.5 |
| PI2 | 170 | 195 | 640 | 797 | 29 | 29.1 |
| PI3 | 169 | 190 | 450 | 800 | 23 | 26.7 |
| Terpolyamides | % WA a | Block Copolymers | % WA a |
|---|---|---|---|
| F2 | 25 | PF2 | 0.1 |
| T2 | 50 | PT2 | 0.5 |
| Monomers | EHOMO (eV) | ELUMO (eV) | Total Energy (a.u.) | Dipole Moment (Debye) | Imaginary Frequency (cm−1) |
|---|---|---|---|---|---|
| M1 | −0.38812 | −0.03531 | −1367.012 | 5.3808 | −21.06 -Cl Bending vibrations |
| M1′ | −0.31173 | −0.13196 | −1371.183 | 1.0527 | -- |
| M2 | −0.33884 | −0.33884 | −188.213 | 0.0086 | −215.20 (-H symmetric bending mode 1) −200.05 (-H symmetric bending mode 2) |
| M3 | −0.20167 | −0.20167 | −1116.536 | 8.8394 | Nil |
| M4 | −0.20966 | −0.00810 | −645.619 | 1.5962 | −624.73 (-HN1 angle bending) −545.52 (-HN2 angle bending) −50.43 (M4 symmetric bending) |
| M5 | −0.17273 | 0.01828 | −609.956 | 2.2372 | -- |
| M6 | 0.10521 | 0.10521 | −1862.783 | 1.2979 | -- |
| M7 | −0.31548 | 0.15462 | −1429.508 | 5.355 | -- |
| F2 | −0.3221 | 0.31617 | −4513.094 | 1.287 | -- |
| T2 | −0.2152 | 0.22139 | −4892.113 | 1.114 | -- |
| PF2 | −0.1872 | 0.35460 | −5732.652 | 4.232 | -- |
| PT2 | −0.2238 | 0.11828 | −6212.342 | 4.338 | -- |
| Monomers | Enthalpy (kcal/mol) | Heat Capacity (Ca/mol-K) | Entropy (Ca/mol/K) | Zero-Point Vibrational Energy (kcal/mol) | Total Thermal Energy (kcal/mol) |
|---|---|---|---|---|---|
| M1 | −1366.896 | 31.845 | 93.541 | 66.735 | 72.017 |
| M1′ | −1371.070 | 37.492 | 104.295 | 63.833 | 70.379 |
| M2 | −188.090 | 15.274 | 68.483 | 72.736 | 75.634 |
| M3 | −1116.295 | 60.650 | 126.775 | 140.955 | 150.697 |
| M4 | −645.388 | 45.903 | 106.120 | 136.397 | 147.474 |
| M5 | −609.696 | 53.31 | 120.813 | 152.976 | 161.469 |
| M6 | −1862.456 | 49.90 | 111.356 | 119.420 | 127.531 |
| M7 | −2165.322 | 48.71 | 152.244 | 172.34 | 132.560 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mushtaq, I.; Jabeen, E.; Akhter, Z.; Javed, F.; Hassan, A.; Khan, M.S.U.; Ullah, F.; Shah, F.U. Ferrocene-Based Terpolyamides and Their PDMS-Containing Block Copolymers: Synthesis and Physical Properties. Polymers 2022, 14, 5087. https://doi.org/10.3390/polym14235087
Mushtaq I, Jabeen E, Akhter Z, Javed F, Hassan A, Khan MSU, Ullah F, Shah FU. Ferrocene-Based Terpolyamides and Their PDMS-Containing Block Copolymers: Synthesis and Physical Properties. Polymers. 2022; 14(23):5087. https://doi.org/10.3390/polym14235087
Chicago/Turabian StyleMushtaq, Irrum, Erum Jabeen, Zareen Akhter, Fatima Javed, Azfar Hassan, Muhammad Saif Ullah Khan, Faheem Ullah, and Faiz Ullah Shah. 2022. "Ferrocene-Based Terpolyamides and Their PDMS-Containing Block Copolymers: Synthesis and Physical Properties" Polymers 14, no. 23: 5087. https://doi.org/10.3390/polym14235087
APA StyleMushtaq, I., Jabeen, E., Akhter, Z., Javed, F., Hassan, A., Khan, M. S. U., Ullah, F., & Shah, F. U. (2022). Ferrocene-Based Terpolyamides and Their PDMS-Containing Block Copolymers: Synthesis and Physical Properties. Polymers, 14(23), 5087. https://doi.org/10.3390/polym14235087