Amphiphilic Molecular Brushes with Regular Polydimethylsiloxane Backbone and Poly-2-isopropyl-2-oxazoline Side Chains. 3. Influence of Grafting Density on Behavior in Organic and Aqueous Solutions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characteristics of Investigated Samples
2.2. Synthesis of Multicenter Macroinitiator PDMS3
2.3. Synthesis of Nonregular Macroinitiator PDMS10
2.4. Polymer Brushes Synthesis
2.5. Investigation of Molecular Dispersed Solution of PDMSk and PDMSk-graft-PiPrOx
2.6. Investigation of Aqueous Solutions of PDMSk-graft-PiPrOx
2.7. Microscopic Investigation
3. Results and Discussion
3.1. Synthesis of Macroinitiator PDMS3
3.2. Synthesis of Macroinitiator PDMS10
3.3. Synthesis of Molecular Brushes
3.4. Structural Characteristics of Macroinitiators and Grafted Copolymers
3.5. Hydrodynamical and Conformational Characteristics of Macroinitiators and Grafted Copolymers
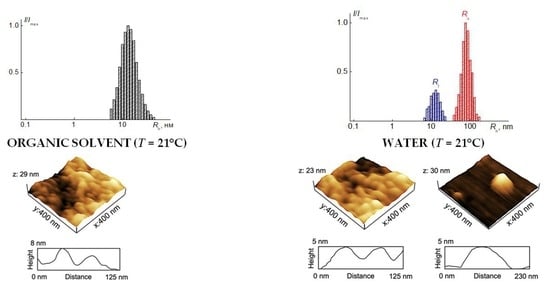
3.6. Self-Organization of Grafted Copolymers in Aqueous Solutions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoogenboom, R.; Schlaad, H. Bioinspired poly(2-oxazoline)s. Polymers 2011, 3, 467–488. [Google Scholar] [CrossRef] [Green Version]
- Appavoo, D.; Park, S.Y.; Zhai, L.J. Responsive polymers for medical diagnostics. J. Mater. Chem. B. 2020, 8, 6217–6232. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.M.; Spencer, D.S.; Peppas, N.A. Advanced architectures in the design of responsive polymers for cancer nanomedicine. J. Appl. Polym. Sci. 2018, 135, 46154. [Google Scholar] [CrossRef] [PubMed]
- Bordat, A.; Boissenot, T.; Nicolas, J.; Tsapis, N. Thermoresponsive polymer nanocarriers for biomedical applications. Adv. Drug Deliv. Rev. 2019, 138, 167–192. [Google Scholar] [CrossRef] [PubMed]
- Sponchioni, M.; Palmiero, U.C.; Moscatelli, D. Thermo-responsive polymers: Applications of smart materials in drug delivery and tissue engineering. Mater. Sci. Eng. C. 2019, 102, 589–605. [Google Scholar] [CrossRef]
- Koyama, M.; Hirano, T.; Ohno, K.; Katsumoto, Y. Molecular Understanding of the UCST-Type Phase Separation Behavior of a Stereocontrolled Poly(N-isopropylacrylamide) in Bis(2-methoxyethyl) Ether. J. Phys. Chem. B 2008, 112, 10854–10860. [Google Scholar] [CrossRef]
- Zhang, Q.; Weber, C.; Schubert, U.S.; Hoogenboom, R. Thermoresponsive polymers with lower critical solution temperature: From fundamental aspects and measuring techniques to recommended turbidimetry conditions. Mater. Horiz. 2017, 4, 109–116. [Google Scholar] [CrossRef]
- Champ, S.; Xue, W.; Huglin, M.B. Concentrating aqueous solutions of water soluble polymers by thermoreversible swelling of poly[(N-isopropylacrylamide)-co-(acrylic acid)] hydrogels. Macromol. Chem. Phys. 2000, 201, 931–940. [Google Scholar] [CrossRef]
- Zhang, X.Z.; Zhuo, R.X. Synthesis and properties of thermosensitive poly(N-isopropylacrylamide-co-methyl methacrylate) hydrogel with rapid response. Mater. Lett. 2002, 52, 5–9. [Google Scholar] [CrossRef]
- Park, J.-S.; Kataoka, K. Precise control of lower critical solution temperature of thermosensitive poly(2-isopropyl-2-oxazoline) via gradient copolymerization with 2-ethyl-2-oxazoline as a hydrophilic comonomer. Macromolecules 2006, 39, 6622–6630. [Google Scholar] [CrossRef]
- Huber, S.; Jordan, R. Modulation of the lower critical solution temperature of 2-Alkyl-2-oxazoline copolymers. Colloid Polym. Sci. 2008, 286, 395–402. [Google Scholar] [CrossRef]
- Lefebvre, M.D.; Olvera de la Cruz, M.; Shull, K.R. Phase Segregation in Gradient Copolymer Melts. Macromolecules 2004, 37, 1118–1123. [Google Scholar] [CrossRef]
- Weber, C.; Hoogenboom, R.; Schubert, U.S. Temperature responsive bio-compatible polymers based on poly(ethylene oxide) and poly(2-oxazoline)s. Progr. Polym. Sci. 2012, 37, 686–714. [Google Scholar] [CrossRef]
- Krumm, C.; Fik, C.P.; Meuris, M.; Dropalla, G.J.; Geltenpoth, H.; Sickmann, A.; Tiller, J.C. Well-defined amphiphilic poly(2-oxazoline) ABA-triblock copolymers and their aggregation behavior in aqueous solution Macromol. Rapid Commun. 2012, 33, 1677–1682. [Google Scholar] [CrossRef]
- Kyriakos, K.; Aravopoulou, D.; Augsbach, L.; Sapper, J.; Ottinger, S.; Psylla, C.; Aghebat Rafat, A.; Benitez-Montoya, C.A.; Miasnikova, A.; Di, Z.; et al. Novel thermoresponsive block copolymers having different architectures—Structural, rheological, thermal, and dielectric investigations. Colloid Polym. Sci. 2014, 292, 1757–1774. [Google Scholar] [CrossRef] [Green Version]
- Steinschulte, A.A.; Schulte, B.; Rütten, S.; Eckert, T.; Okuda, J.; Möller, M.; Schneider, S.; Borisov, O.V.; Plamper, F.A. Effects of architecture on the stability of thermosensitive unimolecular micelles. Phys. Chem. Chem. Phys. 2014, 16, 4917–4932. [Google Scholar] [CrossRef] [Green Version]
- Steinschulte, A.A.; Schulte, B.; Erberich, M.; Borisov, O.V.; Plamper, F.A. Unimolecular Janus Micelles by Microenvironment-Induced, Internal Complexation. ACS Macro Lett. 2012, 1, 504–507. [Google Scholar] [CrossRef]
- Pu, W.-F.; Ushakova, A.; Liu, R.; Lazutin, A.A.; Vasilevskaya, V.V. Self-assembly in amphiphilic spherical brushes. J. Chem. Phys. 2020, 152, 234903. [Google Scholar] [CrossRef]
- Chen, T.; Yang, H.; Wu, X.; Yu, D.; Ma, A.; He, X.; Sun, K.; Wang, J. Ultrahighly charged amphiphilic polymer brushes with super-antibacterial and self-cleaning capabilities. Langmuir 2019, 35, 3031–3037. [Google Scholar] [CrossRef]
- Kizil, S.; Bulbul Sonmez, H. Star PEG-based amphiphilic polymers: Synthesis, characterization and swelling behaviors. Polym. Bull. 2019, 76, 2081–2096. [Google Scholar] [CrossRef]
- Zhang, M.; Müller, A.H.E. Cylindrical polymer brushes. J. Polym. Sci. A Polym. Chem. 2005, 43, 3461–3481. [Google Scholar] [CrossRef]
- Verduzco, R.; Li, X.Y.; Pesek, S.L.; Stein, G.E. Structure, function, self-assembly, and applications of bottlebrush copolymers. Chem. Soc. Rev. 2015, 44, 2405–2420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheiko, S.S.; Sumerlin, B.S.; Matyjaszewski, K. Cylindrical molecular brushes: Synthesis, characterization, and properties. Prog. Polym. Sci. 2008, 33, 759–785. [Google Scholar] [CrossRef]
- Lee, H.; Pietrasik, J.; Sheiko, S.S.; Matyjaszewski, K. Stimuli-responsive molecular brushes. Progr. Polym. Sci. 2010, 35, 24–44. [Google Scholar] [CrossRef]
- Müllner, M.; Lunkenbein, T.; Breu, J.; Caruso, F.; Müller, A.H.E. Template-Directed Synthesis of Silica Nanowires and Nanotubes from Cylindrical Core–Shell Polymer Brushes. Chem. Mater. 2012, 24, 1802–1810. [Google Scholar] [CrossRef]
- Palacios-Hernandez, T.; Luo, H.; Garcia, E.A.; Pacheco, L.A.; Herrera-Alonso, M. Nanoparticles from Amphiphilic Heterografted Macromolecular Brushes with Short Backbones. Macromolecules 2018, 51, 2831–2837. [Google Scholar] [CrossRef]
- Siegwart, D.J.; Oh, J.K.; Matyjaszewski, K. ATRP in the design of functional materials for biomedical applications. Prog. Polym. Sci. 2012, 37, 18–37. [Google Scholar] [CrossRef] [Green Version]
- Ivanov, I.V.; Meleshko, T.K.; Kashina, A.V.; Yakimansky, A.V. Amphiphilic multicomponent molecular brushes. Russ. Chem. Rev. 2019, 88, 1248–1290. [Google Scholar] [CrossRef]
- Ohta, Y.; Yokozawa, T. Chain-growth condensation polymerization for controlled synthesis of polymers. Adv. Polym. Sci. 2013, 262, 191–238. [Google Scholar]
- Peng, S.; Bhushan, B. Smart polymer brushes and their emerging applications. RSC Adv. 2012, 2, 8557–8578. [Google Scholar] [CrossRef]
- Atanase, L.I.; Riess, G. Self-Assembly of Block and Graft Copolymers in Organic Solvents: An Overview of Recent Advances. Polymers 2018, 10, 62. [Google Scholar] [CrossRef]
- Kudryavtseva, A.A.; Kurlykin, M.P.; Tarabukina, E.B.; Tenkovtsev, A.V.; Filippov, A.P. Behavior of thermosensitive graft copolymer with aromatic polyester backbone and poly-2-ethyl-2-oxazoline side chains in aqueous solutions. Int. J. Polym. Anal. Charact. 2017, 22, 526–533. [Google Scholar] [CrossRef]
- Tarabukina, E.; Fatullaev, E.; Krasova, A.; Kurlykin, M.; Tenkovtsev, A.; Sheiko, S.S.; Filippov, A. Synthesis, structure, hydrodynamics and thermoresponsiveness of graft copolymer with aromatic polyester backbone at poly-2-isopropyl-2-oxazoline side chains. Polymers 2020, 12, 2643. [Google Scholar] [CrossRef]
- Li, X.; ShamsiJazeyi, H.; Pesek, S.L.; Agrawal, A.; Hammouda, B.; Verduzco, R. Thermoresponsive PNIPAAM bottlebrush polymers with tailored side-chain length and end-group structure. Soft Matter. 2014, 10, 2008–2015. [Google Scholar] [CrossRef] [Green Version]
- Weber, C.; Wagner, M.; Baykal, D.; Hoeppener, S.; Paulus, R.M.; Festag, G.; Altuntas, E.; Schacher, F.H.; Schubert, U.S. Easy access to amphiphilic heterografted poly(2-oxazoline) comb copolymers. Macromolecules 2013, 46, 5107–5116. [Google Scholar] [CrossRef]
- Bühler, J.; Muth, S.; Fischer, K.; Schmidt, M. Collapse of cylindrical brushes with 2-isopropyloxazoline side chains close to the phase boundary. Macromol. Rapid Commun. 2013, 34, 588–594. [Google Scholar] [CrossRef]
- Weber, C.; Rogers, S.; Vollrath, A.; Hoeppener, S.; Rudolph, T.; Fritz, N.; Hoogenboom, R.; Schubert, U.S. Aqueous solution behavior of comb-shaped poly(2-ethyl-2-oxazoline). J. Polym. Sci. Pol. Chem. 2013, 51, 139–148. [Google Scholar] [CrossRef]
- Zhang, N.; Luxenhofer, R.; Jordan, R. Thermoresponsive poly(2-oxazoline) molecular brushes by living ionic polymerization: Kinetic investigations of pendant chain grafting and cloud point modulation by backbone and side chain length variation. Macromol. Chem. Phys. 2012, 213, 973–981. [Google Scholar] [CrossRef]
- Filippov, A.; Tarabukina, E.; Kudriavtseva, A.; Fatullaev, E.; Kurlykin, M.; Tenkovtsev, A. Molecular brushes with poly-2-ethyl-2-oxazoline side chains and aromatic polyester backbone manifesting double stimuli responsiveness. Colloid Polym. Sci. 2019, 297, 1445–1454. [Google Scholar] [CrossRef]
- Ma, H.; Kim, K.T. Self-Assembly of Bottlebrush Block Copolymers into Triply Periodic Nanostructures in a Dilute Solution. Macromolecules 2020, 53, 711–718. [Google Scholar] [CrossRef]
- Atanase, L.I.; Desbrieres, J.; Riess, G. Micellization of synthetic and polysaccharides-based graft copolymers in aqueous media. Progr. Polym. Sci. 2017, 73, 32–60. [Google Scholar] [CrossRef]
- Rodchenko, S.; Amirova, A.; Milenin, S.; Ryzhkov, A.; Talalaeva, E.; Kalinina, A.; Kurlykin, M.; Tenkovtsev, A.; Filippov, A. Amphiphilic molecular brushes with regular polydimethylsiloxane backbone and poly-2-isopropyl-2-oxazoline side chains. 1. Synthesis, characterization and conformation in solution. Europ. Polym. J. 2020, 140, 110035. [Google Scholar] [CrossRef]
- Rodchenko, S.; Amirova, A.; Kurlykin, M.; Tenkovtsev, A.; Milenin, S.; Filippov, A. Amphiphilic Molecular Brushes with Regular Polydimethylsiloxane Backbone and Poly-2-isopropyl-2-oxazoline Side Chains. 2. Self-Organization in Aqueous Solutions on Heating. Polymers 2021, 13, 31. [Google Scholar] [CrossRef] [PubMed]
- Tsvetkov, V.N. Rigid-Chain Polymers Hydrodynamic and Optical Properties in Solution, 1st ed.; Plenum Press: New York, NY, USA, 1989; pp. 1–512. [Google Scholar]
- Rueb, C.J.; Zukoski, C.F. Rheology of suspensions of weakly attractive particles: Approach to gelation. J. Rheol. 1998, 42, 1451–1476. [Google Scholar] [CrossRef]
- Yamakawa, H. Modern Theory of Polymer Solutions; Harper and Row: New York, NY, USA, 1971. [Google Scholar]
- Talalaeva, E.V.; Kalinina, A.A.; Vasilenko, N.G.; Demchenko, N.V.; Cherkaev, G.V.; Goloveshkin, A.S.; Muzafarov, A.M. Selective formation of 1,5-disodiumoxyhexamethyltrisiloxane in the reaction of dimethylsiloxanes and sodium hydroxide. J. Organomet. Chem. 2020, 906, 1210504. [Google Scholar] [CrossRef]
- Flory, P. Statistical Mechanics of Chain Molecules; Wiley-Interscience: New York, NY, USA, 1989. [Google Scholar]
- Filippov, A.; Kozlov, A.; Tarabukina, E.; Obrezkova, M.; Muzafarov, A. Solution properties of comb-like polymers consisting of dimethylsiloxane monomer units. Polym. Int. 2016, 65, 393–399. [Google Scholar] [CrossRef]
- Grube, M.; Leiske, M.N.; Schubert, U.S.; Nischang, I. POx as an alternative to PEG? A hydrodynamic and light scattering study. Macromolecules 2018, 51, 1905–1916. [Google Scholar] [CrossRef]
- Ye, X.D.; Yang, J.X.; Ambreen, J. Scaling laws between the hydrodynamic parameters and molecular weight of linear poly(2-ethyl-2-oxazoline). RSC Adv. 2013, 3, 15108–15113. [Google Scholar] [CrossRef]
- Chen, C.H.; Wilson, J.; Chen, W.; Davis, R.M.; Riffle, J.S. A light-scattering study of poly(2-alkyl-2-oxazoline)s: Effect of temperature and solvent type. Polymer 1994, 35, 3587–3591. [Google Scholar] [CrossRef]
- Tsvetkov, V.N.; Lavrenko, P.N.; Bushin, S.V. A hydrodynamic invariant of polymeric molecules. Russ. Chem. Rev. 1982, 51, 975–993. [Google Scholar] [CrossRef]
- Tsvetkov, V.N.; Lavrenko, P.N.; Bushin, S.V. Hydrodynamic invariant of polymer-molecules. J. Polym. Sci. Part A Polym. Chem. 1984, 22, 3447–3486. [Google Scholar] [CrossRef]
- Filippov, A.P.; Belyaeva, E.V.; Tarabukina, E.B.; Amirova, A.I. Behavior of hyperbranched polymers in solutions. Polym. Sci. Ser. C. 2011, 53, 107–117. [Google Scholar] [CrossRef]
- Shpyrkov, A.A.; Tarasenko, I.I.; Pankova, G.A.; Il’ina, I.E.; Tarasova, E.V.; Tarabukina, E.B.; Vlasov, G.P.; Filippov, A.P. Molecular mass characteristics and hydrodynamic and conformational properties of hyperbranched poly-L-lysines. Polym. Sci.-A 2009, 51, 250–258. [Google Scholar] [CrossRef]
- Pavlov, G.M.; Korneeva, E.V.; Nepogod’ev, S.A.; Jumel, K.; Harding, S.E. Translational and rotational friction of lactodendrimer molecules in solution. Polym. Sci.-A 1998, 40, 1282–1289. [Google Scholar]
- Crescenzi, V.; Flory, P.J. Configuration of the Poly-(dimethylsiloxane) Chain. II. Unperturbed Dimensions and Specific Solvent Effects. J. Am. Chem. Soc. 1964, 86, 141–146. [Google Scholar] [CrossRef]
- Barry, A.J. Viscometric Investigation of Dimethylsiloxane Polymers. J. Appl. Phys. 1946, 17, 1020–1024. [Google Scholar] [CrossRef]
- Takimoto, H.H.; Forbes, C.T.; Laudenslager, R.K. A viscosity–molecular weight relationship for polydimethylsiloxanes. J. Appl. Polym. Sci. 1961, 5, 153–156. [Google Scholar] [CrossRef]
- Andersson, G.R. A study of the pressure dependence of the partial specific volume of macromolecules in solution by compression measurements in the range. Ark. Kem. 1963, 20, 513–571. [Google Scholar]
- Gubarev, A.S.; Monnery, B.D.; Lezov, A.A.; Sedlacek, O.; Tsvetkov, N.V.; Hoogenboom, R.; Filippov, S.K. Conformational properties of biocompatible poly(2-ethyl-2-oxazoline)s in phosphate buffered saline. Polym. Chem. 2018, 9, 2232–2237. [Google Scholar] [CrossRef] [Green Version]
- Schulz, G.V.; Haug, A. Thermodynamik und Struktur in Lösungen von Polydimethylsiloxanen. Z. Phys. Chem. 1961, 34, 328–345. [Google Scholar] [CrossRef]
- Haug, A.; Meyerhoff, G. Hydrodynamisches verhalten gelöster polydimethylsiloxane. Makromol. Chem. 1962, 53, 91–102. [Google Scholar] [CrossRef]
- Sung, J.H.; Lee, D.C. Molecular shape of poly(2-ethyl-2-oxazoline) chains in THF. Polymer 2001, 42, 5771–5779. [Google Scholar] [CrossRef]
- Filippov, A.P.; Belyaeva, E.V.; Krasova, A.S.; Simonova, M.A.; Meleshko, T.K.; Ilgach, D.M.; Bogorad, N.N.; Yakimansky, A.V.; Larin, S.V.; Darinskii, A.A. Conformations of Molecular Brushes Based on Polyimide and Poly(methyl methacrylate) in Selective Solvents: Experiment and Computer Simulation. Polym. Sci.-A 2014, 4, 393–404. [Google Scholar] [CrossRef]
- Zimm, B.H.; Stockmayer, W.H. The Dimensions of Chain Molecules Containing Branches and Rings. J. Chem. Phys. 1949, 17, 1301–1314. [Google Scholar] [CrossRef]
- Burchard, W. Particle scattering factors of some branched polymers. Macromolecules 1977, 10, 919–927. [Google Scholar] [CrossRef]
- Burchard, W. Static and dynamic light scattering from branched polymers and biopolymers. Adv. Polym. Sci. 1983, 48, 1–124. [Google Scholar]
- Daoud, M.; Cotton, J.P. Star shaped polymers: A model for the conformation and its concentration dependence. J. Phys. 1982, 43, 531–538. [Google Scholar] [CrossRef]
- Kirila, T.; Smirnova, A.; Razina, A.; Tenkovtsev, A.; Filippov, A. Synthesis and Conformational Characteristics of Thermosensitive Star-Shaped Six-Arm Polypeptoids. Polymers 2020, 12, 800. [Google Scholar] [CrossRef] [Green Version]
- Zakharova, N.V.; Simonova, M.A.; Zelinskii, S.N.; Annenkov, V.V.; Filippov, A.P. Synthesis, molecular characteristics, and stimulus-sensitivity of graft copolymer of chitosan and poly(N,N-diethylacrylamide). J. Mol. Liq. 2019, 292, 111355. [Google Scholar] [CrossRef]
- Li, C.; Ge, Z.; Fang, J.; Liu, S. Synthesis and self-assembly of coil-rod double hydrophilic diblock copolymer with dually responsive asymmetric centipede-shaped polymer brush as the rod Segment. Macromolecules 2009, 42, 2916–2924. [Google Scholar] [CrossRef]
- Lian, X.; Wu, D.; Song, X.; Zhao, H. Synthesis and self-assembly of amphiphilic asymmetric macromolecular brushes. Macromolecules 2010, 43, 7434–7445. [Google Scholar] [CrossRef]
- Katsumoto, Y.; Tsuchiizu, A.; Qiu, X.; Winnik, F.M. Dissecting the mechanism of the heat-induced phase separation and crystallization of poly(2-isopropyl-2-oxazoline) in water through vibrational spectroscopy and molecular orbital calculations. Macromolecules 2012, 45, 3531–3541. [Google Scholar] [CrossRef]
- Kratochvil, P. Classical Light Scattering from Polymer Solution; Elsevier: Amsterdam, Netherlands, 1987. [Google Scholar]
- Schärtl, W. Light Scattering from Polymer Solutions and Nanoparticle Dispersions; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Amirova, A.; Rodchenko, S.; Makhmudova, Z.; Cherkaev, G.; Milenin, S.; Tatarinova, E.; Kurlykin, M.; Tenkovtsev, A.; Filippov, A. Synthesis, characterization, and investigation of thermosensitive star-shaped poly(2-isopropyl-2-oxazolines) based on carbosilane dendrimers. Macromol. Chem. Phys. 2017, 218, 1600387. [Google Scholar] [CrossRef]
- Amirova, A.; Rodchenko, S.; Milenin, S.; Tatarinova, E.; Kurlykin, M.; Tenkovtsev, A.; Filippov, A. Influence of a hydrophobic core on thermoresponsive behavior of dendrimer-based star-shaped poly(2-isopropyl-2-oxazoline) in aqueous solutions. J. Polym. Res. 2017, 24, 124. [Google Scholar] [CrossRef]
- Rodchenko, S.; Amirova, A.; Milenin, S.; Kurlykin, M.; Tenkovtsev, A.; Filippov, A. Self-organization of thermosensitive star-shaped poly(2-isopropyl-2-oxazolines) influenced by arm number and generation of carbosilane dendrimer core in aqueous solutions. Colloid Polym. Sci. 2020, 298, 355–363. [Google Scholar] [CrossRef]


















| Title 1 | Mw, g∙mol−1 | Rh-D, nm | dn/dc, cm3∙g−1 | , cm3∙g−1 | [η], cm3∙g−1 | A0∙10−10, Erg∙K−1∙mol−1/3 |
|---|---|---|---|---|---|---|
| PDMS3 | 77,000 | 6.3 | 0.0703 | 0.968 | 24 | 3.1 |
| PDMS3-graft-PiPrOx | 440,000 | 13.0 | 0.1156 | 0.935 | 21 | 1.5 |
| PDMS10 | 10,000 | 2.9 | 0.0756 | 0.979 | 16 | 3.0 |
| PDMS10-graft-PiPrOx | 50,000 | 3.7 | 0.1256 | 0.929 | 9.0 | 2.5 |
| Title 1 | λbb, nm | z | ΔL, nm | Lsc, nm | Lsc/∆L | fsc | ω, Mass% |
|---|---|---|---|---|---|---|---|
| PDMS3 | 0.78 | - | 2.1 | - | - | - | |
| PDMS3-graft-PiPrOx | 0.78 | 0.7 | 1.1 | 9.3 | 8.4 | 76 | 17.5 |
| PDMS10 | 2.6 | - | 2.1 | - | - | - | |
| PDMS10-graft-PiPrOx | 2.6 | 0.6 | 4.3 | 14.2 | 3.3 | 5 | 20.0 |
| Polymer | g* | h | ||
|---|---|---|---|---|
| PDMS | PEtOx | PDMS | PEtOx | |
| PDMS3-graft-PiPrOx | 0.20 | 0.13 | 0.63 | 0.44 |
| PDMS10-graft-PiPrOx | 0.41 | 0.23 | 0.58 | 0.42 |
| Title 1 | z | Mw, g·mol−1 | ΔL, nm | Lsc, nm | Lsc/∆L | fsc | ω, Mass% | LCST, °C |
|---|---|---|---|---|---|---|---|---|
| PDMS3-graft-PiPrOx | 0.7 | 440,000 | 1.11 | 9.3 | 8.4 | 76 | 17.5 | <40 |
| PDMS4-graft-PiPrOx-1 * | 0.6 | 400,000 | 1.73 | 6.7 | 3.9 | 86 | 28.8 | ≤27 |
| PDMS4-graft-PiPrOx-2 * | 0.6 | 700,000 | 1.73 | 12.7 | 7.4 | 86 | 16.2 | ≤37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodchenko, S.; Kurlykin, M.; Tenkovtsev, A.; Milenin, S.; Sokolova, M.; Yakimansky, A.; Filippov, A. Amphiphilic Molecular Brushes with Regular Polydimethylsiloxane Backbone and Poly-2-isopropyl-2-oxazoline Side Chains. 3. Influence of Grafting Density on Behavior in Organic and Aqueous Solutions. Polymers 2022, 14, 5118. https://doi.org/10.3390/polym14235118
Rodchenko S, Kurlykin M, Tenkovtsev A, Milenin S, Sokolova M, Yakimansky A, Filippov A. Amphiphilic Molecular Brushes with Regular Polydimethylsiloxane Backbone and Poly-2-isopropyl-2-oxazoline Side Chains. 3. Influence of Grafting Density on Behavior in Organic and Aqueous Solutions. Polymers. 2022; 14(23):5118. https://doi.org/10.3390/polym14235118
Chicago/Turabian StyleRodchenko, Serafim, Mikhail Kurlykin, Andrey Tenkovtsev, Sergey Milenin, Maria Sokolova, Alexander Yakimansky, and Alexander Filippov. 2022. "Amphiphilic Molecular Brushes with Regular Polydimethylsiloxane Backbone and Poly-2-isopropyl-2-oxazoline Side Chains. 3. Influence of Grafting Density on Behavior in Organic and Aqueous Solutions" Polymers 14, no. 23: 5118. https://doi.org/10.3390/polym14235118
APA StyleRodchenko, S., Kurlykin, M., Tenkovtsev, A., Milenin, S., Sokolova, M., Yakimansky, A., & Filippov, A. (2022). Amphiphilic Molecular Brushes with Regular Polydimethylsiloxane Backbone and Poly-2-isopropyl-2-oxazoline Side Chains. 3. Influence of Grafting Density on Behavior in Organic and Aqueous Solutions. Polymers, 14(23), 5118. https://doi.org/10.3390/polym14235118