Fabrication of Superhydrophobic and Light-Absorbing Polyester Fabric Based on Caffeic Acid
Abstract
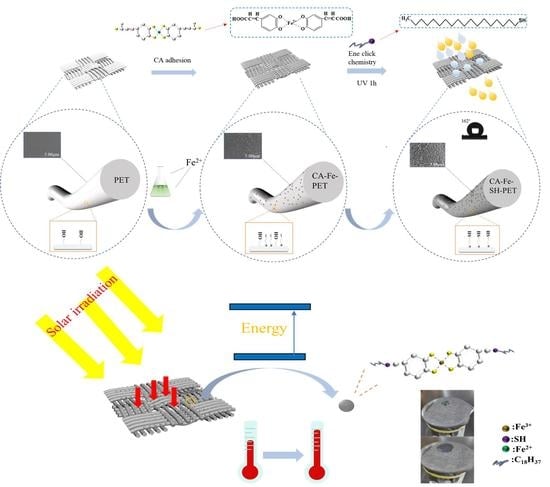
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Superhydrophobic Polyester
2.3. Characterization and Analysis
2.4. Stability Test
2.5. Oil–Water Separation Performance Test
2.6. Photothermal Conversion Performance Test
2.7. UV Resistance Test
3. Results and Discussion
3.1. Surface Morphology and Chemical Structure Characterization
3.2. Stability
3.3. Anti-Fouling and Self-Cleaning Performance of CA-Fe-SH-PET
3.4. Photothermal Performance
3.5. UV Resistance
4. Summary
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Simpson, J.T.; Hunter, S.R.; Aytug, T. Superhydrophobic Materials and Coatings: A Review. Rep. Prog. Phys. 2015, 78, 086501. [Google Scholar] [CrossRef] [PubMed]
- Ellinas, K.; Tserepi, A.; Gogolides, E. Durable Superhydrophobic and Superamphiphobic Polymeric Surfaces and Their Applications: A Review. Adv. Colloid Interface Sci. 2017, 250, 132–157. [Google Scholar] [CrossRef] [PubMed]
- Boreyko, J.B.; Collier, C.P. Delayed Frost Growth on Jumping-Drop Superhydrophobic Surfaces. ACS Nano. 2013, 7, 1618–1627. [Google Scholar] [CrossRef]
- Zeng, Q.; Zhou, H.; Huang, J.; Guo, Z. Review on the Recent Development of Durable Superhydrophobic Materials for Practical Applications. Nanoscale 2021, 13, 11734–11764. [Google Scholar] [CrossRef] [PubMed]
- Elzaabalawy, A.; Verberne, P.; Meguid, S.A. Multifunctional Silica-Silicone Nanocomposite with Regenerative Superhydrophobic Capabilities. ACS Appl. Mater. Interfaces 2019, 11, 42827–42837. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, X.; Shi, J.; Shen, B.; Huang, J.; Hu, J.; Chen, Z.; Lai, Y. A Superhydrophobic TPU/CNTs@SiO2 Coating with Excellent Mechanical Durability and Chemical Stability for Sustainable Anti-Fouling and Anti-Corrosion. Chem. Eng. J. 2022, 434, 134605. [Google Scholar] [CrossRef]
- Ke, C.; Fang, Y.; Zhou, Z.; Wang, G.; Liu, Y.; Wu, W.; Xiao, L.; Zhang, M.; Hu, H.; Liu, J. Superhydrophobic Composite Coating with Excellent Mechanical Durability. Coatings 2022, 12, 185. [Google Scholar] [CrossRef]
- Peng, W.; Gou, X.; Qin, H.; Zhao, M.; Zhao, X.; Guo, Z. Creation of a Multifunctional Superhydrophobic Coating for Composite Insulators. Chem. Eng. J. 2018, 352, 774–781. [Google Scholar] [CrossRef]
- Gong, X.; Zhang, L.; He, S.; Jiang, S.; Wang, W.; Wu, Y. Rewritable Superhydrophobic Coatings Fabricated Using Water-Soluble Polyvinyl Alcohol. Mater. Des. 2020, 196, 109112. [Google Scholar] [CrossRef]
- Oliveira, N.M.; Reis, R.L.; Mano, J.F. Superhydrophobic Surfaces Engineered Using Diatomaceous Earth. ACS Appl. Mater. Interfaces 2013, 5, 4202–4208. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Goyat, M.S.; Hooda, A.; Pandey, J.K.; Kumar, A.; Gupta, R.; Upadhyay, A.K.; Prakash, R.; Kirabira, J.B.; Mandal, P.; et al. Recent Progress in Nano-Oxides and CNTs Based Corrosion Resistant Superhydrophobic Coatings: A Critical Review. Prog. Org. Coat. 2020, 140, 1055121. [Google Scholar] [CrossRef]
- Afrin, S.; Afrin, S.; Fox, D.; Fox, D.; Zhai, L.; Zhai, L.; Zhai, L. Organic Superhydrophobic Coatings with Mechanical and Chemical Robustness. MRS Commun. 2020, 10, 346–352. [Google Scholar] [CrossRef]
- Cheng, J.; Shang, Q.; Liu, C.; Hu, L.; Bo, C.; Hu, Y.; Yang, X.; Zhou, Y.; Lei, W. Fabrication of Cardanol-Based Superhydrophobic Cotton Fabric for Highly Effective Oil-Water Separation. Mater. Today Commun. 2021, 29, 102820. [Google Scholar] [CrossRef]
- Rius-Ayra, O.; Biserova-Tahchieva, A.; López-Jiménez, I.; Llorca-Isern, N. Superhydrophobic and Nanostructured CuFeCo Powder Alloy for the Capture of Microplastics. Colloids Surf. A Physicochem. Eng. Asp. 2021, 627, 127075. [Google Scholar] [CrossRef]
- Szymańska, A.; Przybylak, M.; Maciejewski, H.; Przybylska, A. Thiol-Ene Chemistry as an Effective Tool for Hydrophobization of Cotton Fabrics. Cellulose 2022, 29, 1231–1247. [Google Scholar] [CrossRef]
- Dondoni, A. The Emergence of Thiol-Ene Coupling as a Click Process for Materials and Bioorganic Chemistry. Angew. Chem. Int. Edit. 2008, 47, 8995–8997. [Google Scholar] [CrossRef]
- Xie, A.; Wang, B.; Chen, X.; Wang, Y.; Wang, Y.; Zhu, X.; Xing, T.; Chen, G. Facile Fabrication of Superhydrophobic Polyester Fabric Based on Rapid Oxidation Polymerization of Dopamine for Oil-Water Separation. RSC Adv. 2021, 11, 26992–27002. [Google Scholar] [CrossRef]
- Huang, G.; Huo, L.; Jin, Y.; Yuan, S.; Zhao, R.; Zhao, J.; Li, Z.; Li, Y. Fluorine-Free Superhydrophobic PET Fabric with High Oil Flux for Oil–Water Separation. Prog. Org. Coat. 2022, 163, 106671. [Google Scholar] [CrossRef]
- Fan, J.; Bao, B.; Wang, Z.; Xu, R.; Wang, W.; Yu, D. High Tri-Stimulus Response Photochromic Cotton Fabrics Based on Spiropyran Dye by Thiol-Ene Click Chemistry. Cellulose 2020, 27, 493–510. [Google Scholar] [CrossRef]
- Meng, F.; Muhammad, Y.; Ye, Y.; Ji, J.; Tao, H.; Huang, J.; Zhu, Z.; Li, J. Preparation of Hyperbranch-Structured Polyester Fiber via Thiol-Ene Click Reaction under UV Light Irradiation for Asphalt Binder Modification. J. Appl. Polym. Sci. 2021, 138, 50135. [Google Scholar] [CrossRef]
- Shen, K.; Yu, M.; Li, Q.; Sun, W.; Zhang, X.; Quan, M.; Liu, Z.; Shi, S.; Gong, Y. Synthesis of a Fluorine-Free Polymeric Water-Repellent Agent for Creation of Superhydrophobic Fabrics. Appl. Surf. Sci. 2017, 426, 694–703. [Google Scholar] [CrossRef]
- Qiu, T.; Ge, F.; Li, C.; Lu, S. Study of the thermal degradation of flame-retardant polyester GFRP using TGA and TG-FTIR-GC/MS. J. Therm. Anal. Calorim. 2022, 147, 5743–5760. [Google Scholar] [CrossRef]
- Shcherbakov, A.; Mostovoy, A.; Bekeshev, A.; Burmistrov, I.; Arzamastsev, S.; Lopukhova, M. Effect of Microwave Irradiation at Different Stages of Manufacturing Unsaturated Polyester Nanocomposite. Polymers 2022, 14, 4594. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ou, J.; Fang, X.; Lei, S.; Wang, F.; Li, C.; Li, W.; Hu, Y.; Amirfazli, A.; Wang, P. Robust Superhydrophobic Fabric via UV-Accelerated Atmospheric Deposition of Polydopamine and Silver Nanoparticles for Solar Evaporation and Water/Oil Separation. Chem. Eng. J. 2022, 429, 132539. [Google Scholar] [CrossRef]
- Xue, C.H.; Guo, X.J.; Zhang, M.M.; Ma, J.Z.; Jia, S.T. Fabrication of Robust Superhydrophobic Surfaces by Modification of Chemically Roughened Fibers via Thiol-Ene Click Chemistry. J. Mater. Chem. A Mater. 2015, 3, 21797–21804. [Google Scholar] [CrossRef]
- Xue, C.H.; Fan, Q.Q.; Guo, X.J.; An, Q.F.; Jia, S.T. Fabrication of Superhydrophobic Cotton Fabrics by Grafting of POSS-Based Polymers on Fibers. Appl. Surf. Sci. 2019, 465, 241–248. [Google Scholar] [CrossRef]
- Liao, X.; Li, H.; Zhang, L.; Su, X.; Lai, X.; Zeng, X. Superhydrophobic MGO/PDMS Hybrid Coating on Polyester Fabric for Oil/Water Separation. Prog. Org. Coat. 2018, 115, 172–180. [Google Scholar] [CrossRef]
- Shang, Q.; Liu, C.; Chen, J.; Yang, X.; Hu, Y.; Hu, L.; Zhou, Y.; Ren, X. Sustainable and Robust Superhydrophobic Cotton Fabrics Coated with Castor Oil-Based Nanocomposites for Effective Oil-Water Separation. ACS Sustain. Chem. Eng. 2020, 8, 7423–7435. [Google Scholar] [CrossRef]
- Pakdel, E.; Xie, W.; Wang, J.; Kashi, S.; Sharp, J.; Zhang, Q.; Varley, R.J.; Sun, L.; Wang, X. Superhydrophobic Natural Melanin-Coated Cotton with Excellent UV Protection and Personal Thermal Management Functionality. Chem. Eng. J. 2022, 433, 133688. [Google Scholar] [CrossRef]
- Zeng, H.; Wang, P.; Liang, L.; Hu, H.; Peng, Y.; Li, X.; Liu, C. Facile Preparation of Superhydrophobic Cotton Fabric with a Photothermal Conversion Effect via Polypyrrole Deposition for Oil/Water Separation. J. Environ. Chem. Eng. 2022, 10, 106915. [Google Scholar] [CrossRef]
- Miao, S.; Xiong, Z.; Zhang, J.; Wu, Y.; Gong, X. Polydopamine/SiO2 Hybrid Structured Superamphiphobic Fabrics with Good Photothermal Behavior. Langmuir 2022, 38, 9431–9440. [Google Scholar] [CrossRef] [PubMed]













| Atomic Content (%) | PET | CA -PET | CA-Fe-PET | SH-PET | CA-Fe-SH-PET |
|---|---|---|---|---|---|
| C1s | 74.8 | 71.24 | 71.23 | 79.83 | 85.04 |
| Fe2p | 1.29 | 0.56 | |||
| S2p | 3.03 | 3.69 |
| Property | Original PET Fabric | CA-Fe-SH-PET Fabric |
|---|---|---|
| Breaking strength (N) | 382.48 ± 25.50 (weft) 392.54 ± 20.41 (warp) | 370.74 ± 16.24 (weft) 581.55 ± 11.86 (warp) |
| Elongation at break (%) | 16.27 ± 0.81 (weft) 17.08 ± 0.32 (warp) | 16.52 ± 0.77 (weft) 14.43 ± 0.22 (warp) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, X.; Xie, A.; Yuan, X.; Hou, X.; Lu, J.; Liu, P.; Xiang, Z.; Chen, G.; Xing, T. Fabrication of Superhydrophobic and Light-Absorbing Polyester Fabric Based on Caffeic Acid. Polymers 2022, 14, 5536. https://doi.org/10.3390/polym14245536
Lei X, Xie A, Yuan X, Hou X, Lu J, Liu P, Xiang Z, Chen G, Xing T. Fabrication of Superhydrophobic and Light-Absorbing Polyester Fabric Based on Caffeic Acid. Polymers. 2022; 14(24):5536. https://doi.org/10.3390/polym14245536
Chicago/Turabian StyleLei, Xue, Ailing Xie, Xinya Yuan, Xueni Hou, Jiaosheng Lu, Ping Liu, Zhonglin Xiang, Guoqiang Chen, and Tieling Xing. 2022. "Fabrication of Superhydrophobic and Light-Absorbing Polyester Fabric Based on Caffeic Acid" Polymers 14, no. 24: 5536. https://doi.org/10.3390/polym14245536
APA StyleLei, X., Xie, A., Yuan, X., Hou, X., Lu, J., Liu, P., Xiang, Z., Chen, G., & Xing, T. (2022). Fabrication of Superhydrophobic and Light-Absorbing Polyester Fabric Based on Caffeic Acid. Polymers, 14(24), 5536. https://doi.org/10.3390/polym14245536