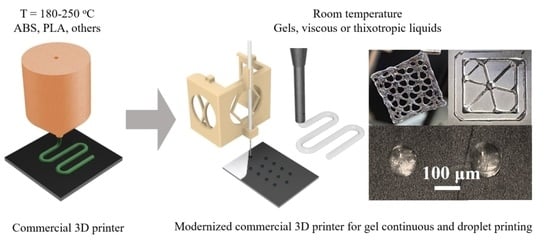
Modification of Commercial 3D Fused Deposition Modeling Printer for Extrusion Printing of Hydrogels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Chemicals
2.1.2. Materials
2.2. Ink Preparation
2.2.1. Inks for Droplet Printing
2.2.2. Inks for Continuous Printing
2.3. Ink Characterization
2.3.1. Rheological Properties
2.3.2. Contact Angle
2.3.3. Attenuated Total Reflectance-Fourier Transform Infrared Spectroscopy (ATR-FTIR Spectroscopy)
2.3.4. Elemental Analysis
2.4. Coding
2.5. Printer Modification
2.6. Drop Printing
2.7. Continuous Printing
3. Results
3.1. Modification of Commercial 3D FDM Printer
3.1.1. Setup
3.1.2. Plunger
3.1.3. Connections
3.1.4. Coding
- (1)
- Drop volume, a parameter applied for every drop is not a straight value, and is measured in arbitrary units, one of which is equal to 0.1 π of the lead screw rod (Figure 2A, 5) rotation. The screw rod rotation transforms to linear motion of the syringe piston, and the lead (i.e., the coefficient of transformation from one full rotation to linear motion) depends on the number of thread systems and thread pitch (thickness of thread). In our case, the lead is equal to 2 mm, thus 10 au results in 1 mm of piston propulsion (in forehead expression set as ‘l’). The final volume, V, should be calculated based on piston radius, r, according to the Equation (1):
- (2)
- The ‘Drop map’ is a block of parameters which correlates with internal function ‘location_function’ in the calculation file of the code. In our case, to describe a drop location map, one should enter the amount of columns and amount of rows of a chess-like map, and set the horizontal shift and vertical shift correspondingly. Overall, these four parameters are combined in Figure 3 as ‘drop map’. If a more complex pattern is needed, the location_function has to be changed in both of its parameters.
- (3)
- Starting_point_coordinates_x_y and z_coordinate_when_touching_pattern are straight values that state the initial position of a sample on a table. Coordinates of sample location on the printing table are entered in pair (x, y), and separated z as a height.
- (4)
- Printer speed is a straight value that shows is the maximal achievable amount of mm per second for the printhead. The real speed of the printhead is affected by two more sets of parameters, particularly acceleration and jerk. Acceleration states how fast the changing of the printhead occurs, and the jerk is maximum instantaneous velocity change. For that, we set limits on the jerks and accelerations in the Constructor block, and these parameters are not implied to be changed. We set them to achieve maximal smoothness when the jerk for movement is 0, so no sharp movement is allowed, and the acceleration is of a relatively small value of 500 mm/s2 in order to minimize vibrations (ten times less than for average printing). The operating system information consists of two variables: the filename and address (folder in C:/). If file with such a name exists, it would be overwritten.
3.2. Inks Rheology
3.2.1. Inks for Droplet Printing
3.2.2. Inks for Continuous Printing
3.3. Extrusion Printing of Hydrogels
3.3.1. Droplet Printing
3.3.2. Continuous Printing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patra, S.; Young, V. A Review of 3D Printing Techniques and the Future in Biofabrication of Bioprinted Tissue. Cell Biochem. Biophys. 2016, 74, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Conner, B.P.; Manogharan, G.P.; Martof, A.N.; Rodomsky, L.M.; Rodomsky, C.M.; Jordan, D.C.; Limperos, J.W. Making Sense of 3-D Printing: Creating a Map of Additive Manufacturing Products and Services. Addit. Manuf. 2014, 4, 64–76. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, Y.S.; Heinrich, M.A.; De Ferrari, F.; Jang, H.L.; Bakht, S.M.; Alvarez, M.M.; Yang, J.; Li, Y.-C.; Trujillo-de-Santiago, G.; et al. Rapid Continuous Multimaterial Extrusion Bioprinting. Adv. Mater. 2017, 29, 1604630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popov, V.V.; Muller-Kamskii, G.; Kovalevsky, A.; Dzhenzhera, G.; Strokin, E.; Kolomiets, A.; Ramon, J. Design and 3D-printing of titanium bone implants: Brief review of approach and clinical cases. Biomed. Eng. Lett. 2018, 8, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Morozova, S.M.; Statsenko, T.G.; Ryabchenko, E.O.; Gevorkian, A.; Adibnia, V.; Lozhkin, M.S.; Kireynov, A.V.; Kumacheva, E. Multicolored Nanocolloidal Hydrogel Inks. Adv. Funct. Mater. 2021, 31, 2105470. [Google Scholar] [CrossRef]
- Prince, E.; Alizadehgiashi, M.; Campbell, M.; Khuu, N.; Albulescu, A.; De France, K.; Ratkov, D.; Li, Y.; Hoare, T.; Kumacheva, E. Patterning of Structurally Anisotropic Composite Hydrogel Sheets. Biomacromolecules 2018, 19, 1276–1284. [Google Scholar] [CrossRef]
- Gevorkian, A.; Morozova, S.M.; Kheiri, S.; Khuu, N.; Chen, H.; Young, E.; Yan, N.; Kumacheva, E. Actuation of Three-Dimensional-Printed Nanocolloidal Hydrogel with Structural Anisotropy. Adv. Funct. Mater. 2021, 31, 2010743. [Google Scholar] [CrossRef]
- Jakus, A.E.; Secor, E.B.; Rutz, A.L.; Jordan, S.W.; Hersam, M.C.; Shah, R.N. Three-Dimensional Printing of High-Content Graphene Scaffolds for Electronic and Biomedical Applications. ACS Nano 2015, 9, 4636–4648. [Google Scholar] [CrossRef]
- De France, K.J.; Yager, K.G.; Chan, K.J.W.; Corbett, B.; Cranston, E.D.; Hoare, T. Injectable Anisotropic Nanocomposite Hydrogels Direct in Situ Growth and Alignment of Myotubes. Nano Lett. 2017, 17, 6487–6495. [Google Scholar] [CrossRef]
- Wilson, S.A.; Cross, L.M.; Peak, C.W.; Gaharwar, A.K. Shear-Thinning and Thermo-Reversible Nanoengineered Inks for 3D Bioprinting. ACS Appl. Mater. Interfaces 2017, 9, 43449–43458. [Google Scholar] [CrossRef]
- Carpio, M.B.; Dabaghi, M.; Ungureanu, J.; Kolb, M.R.; Hirota, J.A.; Moran-Mirabal, J.M. 3D Bioprinting Strategies, Challenges, and Opportunities to Model the Lung Tissue Microenvironment and Its Function. Front. Bioeng. Biotechnol. 2021, 9, 773511. [Google Scholar] [CrossRef] [PubMed]
- Wickramasinghe, S.; Do, T.; Tran, P. FDM-Based 3D Printing of Polymer and Associated Composite: A Review on Mechanical Properties, Defects and Treatments. Polymers 2020, 12, 1529. [Google Scholar] [CrossRef]
- Hassan, K.; Nine, J.; Tung, T.T.; Stanley, N.; Yap, P.L.; Rastin, H.; Yu, L.; Losic, D. Functional inks and extrusion-based 3D printing of 2D materials: A review of current research and applications. Nanoscale 2020, 12, 19007–19042. [Google Scholar] [CrossRef] [PubMed]
- Derakhshanfar, S.; Mbeleck, R.; Xu, K.; Zhang, X.; Zhong, W.; Xing, M. 3D bioprinting for biomedical devices and tissue engineering: A review of recent trends and advances. Bioact. Mater. 2018, 3, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Zhang, Y. Colloidal nanoparticle inks for printing functional devices: Emerging trends and future prospects. J. Mater. Chem. A 2019, 7, 23301–23336. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, D.; Liu, B.; Nian, G.; Li, X.; Yin, J.; Qu, S.; Yang, W. 3D Printing of Multifunctional Hydrogels. Adv. Funct. Mater. 2019, 29, 1900971. [Google Scholar] [CrossRef]
- Khuu, N.; Alizadehgiashi, M.; Gevorkian, A.; Galati, E.; Yan, N.; Kumacheva, E. Temperature-Mediated Microfluidic Extrusion of Structurally Anisotropic Hydrogels. Adv. Mater. Technol. 2019, 4, 1800627. [Google Scholar] [CrossRef]
- Gonzalez-Fernandez, T.; Tenorio, A.J.; Campbell, K.T.; Silva, E.A.; Leach, J.K. Alginate-Based Bioinks for 3D Bioprinting and Fabrication of Anatomically Accurate Bone Grafts. Tissue Eng. Part A 2021, 27, 1168–1181. [Google Scholar] [CrossRef]
- Zhang, H.; Qi, T.; Zhu, X.; Zhou, L.; Li, Z.; Zhang, Y.-F.; Yang, W.; Yang, J.; Peng, Z.; Zhang, G.; et al. 3D Printing of a PDMS Cylindrical Microlens Array with 100% Fill-Factor. ACS Appl. Mater. Interfaces 2021, 13, 36295–36306. [Google Scholar] [CrossRef]
- Kudryavtseva, V.; Bukatin, A.; Vyacheslavova, E.; Gould, D.; Sukhorukov, G.B. Printed asymmetric microcapsules: Facile loading and multiple stimuli-responsiveness. Biomater. Adv. 2022, 136, 212762. [Google Scholar] [CrossRef]
- Guselnikova, O.; Postnikov, P.S.; Trelin, A.; Švorčík, V.; Lyutakov, O. Dual Mode Chip Enantioselective Express Discrimination of Chiral Amines via Wettability-Based Mobile Application and Portable Surface-Enhanced Raman Spectroscopy Measurements. ACS Sensors 2019, 4, 1032–1039. [Google Scholar] [CrossRef] [PubMed]
- del Burgo, L.S.; Ciriza, J.; Noguera, A.E.; Illa, X.; Cabruja, E.; Orive, G.; Hernandez, R.M.; Villa, R.; Pedraz, J.L.; Alvarez, M. 3D Printed porous polyamide macrocapsule combined with alginate microcapsules for safer cell-based therapies. Sci. Rep. 2018, 8, 8512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, C.; Pascall, A.J.; Dudukovic, N.; Worsley, M.A.; Kuntz, J.D.; Duoss, E.B.; Spadaccini, C.M. Colloidal Materials for 3D Printing. Annu. Rev. Chem. Biomol. Eng. 2019, 10, 17–42. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Jambhulkar, S.; Ravichandran, D.; Zhu, Y.; Kakarla, M.; Nian, Q.; Azeredo, B.; Chen, X.; Jin, K.; Vernon, B.; et al. 3D Printing-Enabled Nanoparticle Alignment: A Review of Mechanisms and Applications. Small 2021, 17, 2100817. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, J.; Yao, Q.; Ji, C.; Liu, J.; Zhu, Q. 3D printing with cellulose materials. Cellulose 2018, 25, 4275–4301. [Google Scholar] [CrossRef]
- Cao, L.; Lu, W.; Mata, A.; Nishinari, K.; Fang, Y. Egg-box model-based gelation of alginate and pectin: A review. Carbohydr. Polym. 2020, 242, 116389. [Google Scholar] [CrossRef]
- Neterebskaia, V.O.; Goncharenko, A.O.; Morozova, S.M.; Kolchanov, D.S.; Vinogradov, A.V. Inkjet Printing Humidity Sensing Pattern Based on Self-Organizing Polystyrene Spheres. Nanomaterials 2020, 10, 1538. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koltsov, S.I.; Statsenko, T.G.; Morozova, S.M. Modification of Commercial 3D Fused Deposition Modeling Printer for Extrusion Printing of Hydrogels. Polymers 2022, 14, 5539. https://doi.org/10.3390/polym14245539
Koltsov SI, Statsenko TG, Morozova SM. Modification of Commercial 3D Fused Deposition Modeling Printer for Extrusion Printing of Hydrogels. Polymers. 2022; 14(24):5539. https://doi.org/10.3390/polym14245539
Chicago/Turabian StyleKoltsov, Semyon I., Tatiana G. Statsenko, and Sofia M. Morozova. 2022. "Modification of Commercial 3D Fused Deposition Modeling Printer for Extrusion Printing of Hydrogels" Polymers 14, no. 24: 5539. https://doi.org/10.3390/polym14245539
APA StyleKoltsov, S. I., Statsenko, T. G., & Morozova, S. M. (2022). Modification of Commercial 3D Fused Deposition Modeling Printer for Extrusion Printing of Hydrogels. Polymers, 14(24), 5539. https://doi.org/10.3390/polym14245539