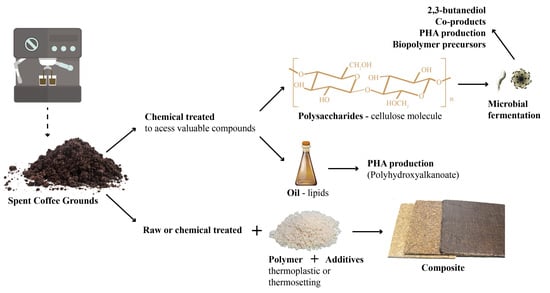
Valorization of Spent Coffee Grounds as Precursors for Biopolymers and Composite Production
Abstract
:1. Introduction
2. Polysaccharide Extraction from SCG
3. Conversion of Polysaccharides and Their Hydrolysates into Biopolymer Precursors
3.1. 2,3-Butanediol Synthesis
| Lignocellulosic Biomass | Production Method | Type of Sugar | 2,3-BD Yield | References |
|---|---|---|---|---|
| Mixed biomass | Hydrolyses and flask fermentation by S. cerevisiae | Xylose | 0.27 g/g | [49] |
| Sorghum biomass and wood | Hydrolyses and shaken flask, followed by bioreactor fermentation by B. licheniformis | Glucose and Xylose | 0.45 g/g 0.40 g/g | [50] |
| Corncob | Alkali pretreatment, hydrolyses, and batch/fed-batch fermentation by E. cloacae | Glucose and Xylose | 0.42 g/g | [51] |
| Kenaf core | Calcium peroxide pretreatment, hydrolyses, and batch fermentation by K. pneumoniae | Glucose and Xylose | 0.38 g/g | [52] |
| Sunflower and pine tree | Hydrolyses and shaken flask fermentation by K. oxytoca | Glucose, Xylose, Galactose, and Mannose | 0.29 g/g 0.22 g/g | [53] |
| Sugar cane bagasse | Hydrolyses and fed-batch fermentation by E. ludwigii | Xylose | 0.38 g/g | [54] |
| Brewer’s spent grain | Microwave-assisted alkali pretreatment, hydrolyses, and shaken flask fermentation by E. ludwigii | Glucose | 0.48 g/g | [55] |
3.1.1. Conversion of 2,3-BD into Biopolymer Precursors
3.1.2. Valorization of the Coproducts from 2,3-Butanediol Synthesis
3.2. Polyhydroxyalkanoate (PHA) Synthesis
3.3. Other Biopolymer Precursors
4. SCG Oil
5. SCG Polymer Composites
5.1. Polythylene (PE) Composites
5.2. Polypropylene (PP) Composites
5.3. Polyurethane (PU) Composites
5.4. Poly(Lactic Acid) (PLA) Composites
5.5. Poly(Butylene Adipate-Co-Terephthalate) (PBAT) Composites
5.6. Polyvinyl Alcohol (PVA) Composites
5.7. Epoxy Composites
5.8. Rubber Composites
6. SCG Reuse Routes
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wu, C.T.; Agrawal, D.C.; Huang, W.Y.; Hsu, H.C.; Yang, S.J.; Huang, S.L.; Lin, Y.S. Functionality Analysis of Spent Coffee Ground Extracts Obtained by the Hydrothermal Method. J. Chem. 2019, 2019, 4671438. [Google Scholar] [CrossRef] [Green Version]
- Garcia, C.V.; Kim, Y.T. Spent Coffee Grounds and Coffee Silverskin as Potential Materials for Packaging: A Review. J. Polym. Environ. 2021, 29, 2372–2384. [Google Scholar] [CrossRef]
- Kourmentza, C.; Economou, C.N.; Tsafrakidou, P.; Kornaros, M. Spent coffee grounds make much more than waste: Exploring recent advances and future exploitation strategies for the valorization of an emerging food waste stream. J. Clean. Prod. 2018, 172, 980–992. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Machado, E.M.S.; Martins, S.; Teixeira, J.A. Production, Composition, and Application of Coffee and Its Industrial Residues. Food Bioprocess Technol. 2011, 4, 661–672. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Yang, J.; Wang, S.; Rupasinghe, H.P.V.; He, Q. (Sophia) Experimental exploration of processes for deriving multiple products from spent coffee grounds. Food Bioprod. Process. 2021, 128, 21–29. [Google Scholar] [CrossRef]
- Atabani, A.E.; Mercimek, S.M.; Arvindnarayan, S.; Shobana, S.; Kumar, G.; Cadir, M.; Al-Muhatseb, A.H. Valorization of spent coffee grounds recycling as a potential alternative fuel resource in Turkey: An experimental study. J. Air Waste Manag. Assoc. 2017, 68, 196–214. [Google Scholar] [CrossRef] [Green Version]
- Soares, B.; Gama, N.; Freire, C.S.R.; Barros-Timmons, A.; Brandão, I.; Silva, R.; Neto, C.P.; Ferreira, A. Spent coffee grounds as a renewable source for ecopolyols production. J. Chem. Technol. Biotechnol. 2015, 90, 1480–1488. [Google Scholar] [CrossRef]
- Kovalcik, A.; Obruca, S.; Marova, I. Valorization of spent coffee grounds: A review. Food Bioprod. Process. 2018, 110, 104–119. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Teixeira, J.A.; Mussatto, S.I. Chemical, Functional and Structural Properties of Spent Coffee Grounds and Coffee Silverskin. Food Bioprocess. Technol. 2014, 7, 3493–3503. [Google Scholar] [CrossRef] [Green Version]
- Hudeckova, H.; Neureiter, M.; Obruca, S.; Frühauf, S.; Marova, I. Biotechnological conversion of spent coffee grounds into lactic acid. Lett. Appl. Microbiol. 2018, 66, 306–312. [Google Scholar] [CrossRef]
- Saratale, G.D.; Bhosale, R.; Shobana, S.; Banu, J.R.; Pugazhendhi, A.; Mahmoud, E.; Sirohi, R.; Kant Bhatia, S.; Atabani, A.E.; Mulone, V.; et al. A review on valorization of spent coffee grounds (SCG) towards biopolymers and biocatalysts production. Bioresour. Technol. 2020, 314, 123800. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yuan, W.; Lu, Y.; Liu, S.Q. Biotransformation of spent coffee grounds by fermentation with monocultures of Saccharomyces cerevisiae and Lachancea thermotolerans aided by yeast extracts. LWT 2021, 138, 110751. [Google Scholar] [CrossRef]
- Battista, F.; Barampouti, E.M.; Mai, S.; Bolzonella, D.; Malamis, D.; Moustakas, K.; Loizidou, M. Added-value molecules recovery and biofuels production from spent coffee grounds. Renew. Sustain. Energy Rev. 2020, 131, 110007. [Google Scholar] [CrossRef]
- Kwon, E.E.; Yi, H.; Jeon, Y.J. Sequential co-production of biodiesel and bioethanol with spent coffee grounds. Bioresour. Technol. 2013, 136, 475–480. [Google Scholar] [CrossRef]
- Auguścik-Królikowska, M.; Ryszkowska, J.; Ambroziak, A.; Szczepkowski, L.; Oliwa, R.; Oleksy, M. The structure and properties of viscoelastic polyurethane foams with fillers from coffee grounds. Polimery 2020, 65, 708–718. [Google Scholar] [CrossRef]
- Sohn, J.S.; Ryu, Y.; Yun, C.S.; Zhu, K.; Cha, S.W. Extrusion compounding process for the development of eco-friendly SCG/PP composite pellets. Sustainability 2019, 11, 1720. [Google Scholar] [CrossRef] [Green Version]
- García-García, D.; Carbonell, A.; Samper, M.D.; García-Sanoguera, D.; Balart, R. Green composites based on polypropylene matrix and hydrophobized spend coffee ground (SCG) powder. Compos. Part B Eng. 2015, 78, 256–265. [Google Scholar] [CrossRef]
- Essabir, H.; Raji, M.; Laaziz, S.A.; Rodrique, D.; Bouhfid, R.; Qaiss, A.E.K. Thermo-mechanical performances of polypropylene biocomposites based on untreated, treated and compatibilized spent coffee grounds. Compos. Part B 2018, 149, 1–11. [Google Scholar] [CrossRef]
- Songtipya, L.; Limchu, T.; Phuttharak, S.; Songtipya, P.; Kalkornsurapranee, E. Poly(lactic acid)-based Composites Incorporated with Spent Coffee Ground and Tea Leave for Food Packaging Application: A Waste to Wealth. IOP Conf. Ser. Mater. Sci. Eng. 2019, 553. [Google Scholar] [CrossRef]
- da Silva, A.P.; de Pereira, M.P.; Passador, F.R.; Montagna, L.S. PLA/Coffee Grounds Composites: A Study of Photodegradation and Biodegradation in Soil. Macromol. Symp. 2020, 394, 1–9. [Google Scholar] [CrossRef]
- Arrigo, R.; Bartoli, M.; Malucelli, G. Poly(lactic Acid)–Biochar Biocomposites: Effect of Processing and Filler Content on Rheological, Thermal, and Mechanical Properties. Polymers 2020, 12, 892. [Google Scholar] [CrossRef] [Green Version]
- McNutt, J.; He, Q. (Sophia) Spent coffee grounds: A review on current utilization. J. Ind. Eng. Chem. 2019, 71, 78–88. [Google Scholar] [CrossRef]
- Wu, C.S. Renewable resource-based green composites of surface-treated spent coffee grounds and polylactide: Characterisation and biodegradability. Polym. Degrad. Stab. 2015, 121, 51–59. [Google Scholar] [CrossRef]
- Campos-Vega, R.; Loarca-Piña, G.; Vergara-Castañeda, H.A.; Dave Oomah, B. Spent coffee grounds: A review on current research and future prospects. Trends Food Sci. Technol. 2015, 45, 24–36. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Carneiro, L.M.; Silva, J.P.A.; Roberto, I.C.; Teixeira, J.A. A study on chemical constituents and sugars extraction from spent coffee grounds. Carbohydr. Polym. 2011, 83, 368–374. [Google Scholar] [CrossRef] [Green Version]
- Simões, J.; Madureira, P.; Nunes, F.M.; do Rosário Domingues Domingues, M.; Vilanova, M.; Coimbra, M.A. Immunostimulatory properties of coffee mannans. Mol. Nutr. Food Res. 2009, 53, 1036–1043. [Google Scholar] [CrossRef]
- Fischer, M.; Reimann, S.; Trovato, V.; Redgwell, R.J. Polysaccharides of green Arabica and Robusta coffee beans. Carbohydr. Res. 2001, 330, 93–101. [Google Scholar] [CrossRef]
- Simões, J.; Nunes, F.M.; Domingues, M.; do Rosário Domingues, M.; Coimbra, M.A. Structural features of partially acetylated coffee galactomannans presenting immunostimulatory activity. Carbohydr. Polym. 2010, 79, 397–402. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Cerqueira, M.A.; Teixeira, J.A.; Mussatto, S.I. Characterization of polysaccharides extracted from spent coffee grounds by alkali pretreatment. Carbohydr. Polym. 2015, 127, 347–354. [Google Scholar] [CrossRef] [Green Version]
- Simões, J.; Nunes, F.M.; Domingues, M.R.; Coimbra, M.A. Extractability and structure of spent coffee ground polysaccharides by roasting pre-treatments. Carbohydr. Polym. 2013, 97, 81–89. [Google Scholar] [CrossRef]
- Wyman, C.E.; Decker, S.R.; Himmel, M.E.; Brady, J.W.; Skopec, C.E.; Viikari, L. Acid Hydrolysis of Cellulose and Hemicellulose, 2nd ed.; Marcel Dekker: New York, NY, USA, 2005. [Google Scholar]
- Dos Santos, L.C.; Adarme, O.F.H.; Baêta, B.E.L.; Gurgel, L.V.A.; de Aquino, S.F. Production of biogas (methane and hydrogen) from anaerobic digestion of hemicellulosic hydrolysate generated in the oxidative pretreatment of coffee husks. Bioresour. Technol. 2018, 263, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Batista, M.J.P.A.; Ávila, A.F.; Franca, A.S.; Oliveira, L.S. Polysaccharide-rich fraction of spent coffee grounds as promising biomaterial for films fabrication. Carbohydr. Polym. 2020, 233, 115851. [Google Scholar] [CrossRef] [PubMed]
- Mussatto, S.I.; Roberto, I.C. Acid hydrolysis and fermentation of brewer’s spent grain to produce xylitol. J. Sci. Food Agric. 2005, 85, 2453–2460. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Teixeira, J.A.; Mussatto, S.I. Extraction of polysaccharides by autohydrolysis of spent coffee grounds and evaluation of their antioxidant activity. Carbohydr. Polym. 2017, 157, 258–266. [Google Scholar] [CrossRef] [Green Version]
- Getachew, A.T.; Cho, Y.J.; Chun, B.S. Effect of pretreatments on isolation of bioactive polysaccharides from spent coffee grounds using subcritical water. Int. J. Biol. Macromol. 2018, 109, 711–719. [Google Scholar] [CrossRef]
- Passos, C.P.; Rudnitskaya, A.; Neves, J.M.M.G.C.; Lopes, G.R.; Evtuguin, D.V.; Coimbra, M.A. Structural features of spent coffee grounds water-soluble polysaccharides: Towards tailor-made microwave assisted extractions. Carbohydr. Polym. 2019, 214, 53–61. [Google Scholar] [CrossRef]
- Passos, C.P.; Coimbra, M.A. Microwave superheated water extraction of polysaccharides from spent coffee grounds. Carbohydr. Polym. 2013, 94, 626–633. [Google Scholar] [CrossRef]
- Ji, X.J.; Huang, H.; Ouyang, P.K. Microbial 2,3-butanediol production: A state-of-the-art review. Biotechnol. Adv. 2011, 29, 351–364. [Google Scholar] [CrossRef]
- Hakizimana, O.; Matabaro, E.; Lee, B.H. The current strategies and parameters for the enhanced microbial production of 2,3-butanediol. Biotechnol. Rep. 2020, 25, e00397. [Google Scholar] [CrossRef]
- Celińska, E.; Grajek, W. Biotechnological production of 2,3-butanediol—Current state and prospects. Biotechnol. Adv. 2009, 27, 715–725. [Google Scholar] [CrossRef]
- Song, C.W.; Park, J.M.; Chung, S.C.; Lee, S.Y.; Song, H. Microbial production of 2,3-butanediol for industrial applications. J. Ind. Microbiol. Biotechnol. 2019, 46, 1583–1601. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S.; Khairul Islam, M.; Khalid Khanzada, N.; Kyoungjin An, A.; Chaiprapat, S.; Leu, S.Y. Whole sugar 2,3-butanediol fermentation for oil palm empty fruit bunches biorefinery by a newly isolated Klebsiella pneumoniae PM2. Bioresour. Technol. 2021, 333, 125206. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Li, Z.G.; Dai, J.Y.; Zhang, D.J.; Xiu, Z.L. Aqueous two-phase extraction of 2,3-butanediol from fermentation broths using an ethanol/phosphate system. Process Biochem. 2009, 44, 112–117. [Google Scholar] [CrossRef]
- Syu, M.J. Biological production of 2,3-butanediol. Appl. Microbiol. Biotechnol. 2001, 55, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Hazeena, S.H.; Sindhu, R.; Pandey, A.; Binod, P. Lignocellulosic bio-refinery approach for microbial 2,3-Butanediol production. Bioresour. Technol. 2020, 302, 122873. [Google Scholar] [CrossRef]
- Ribeiro, L.S.; da Cruz Pedrozo Miguel, M.G.; Martinez, S.J.; Bressani, A.P.P.; Evangelista, S.R.; Batista, C.F.S.E.; Schwan, R.F. The use of mesophilic and lactic acid bacteria strains as starter cultures for improvement of coffee beans wet fermentation. World J. Microbiol. Biotechnol. 2020, 36, 1–15. [Google Scholar] [CrossRef]
- Lee, L.W.; Cheong, M.W.; Curran, P.; Yu, B.; Liu, S.Q. Modulation of coffee aroma via the fermentation of green coffee beans with Rhizopus oligosporus: I. Green coffee. Food Chem. 2016, 211, 916–924. [Google Scholar] [CrossRef]
- Kim, S.J.; Seo, S.O.; Park, Y.C.; Jin, Y.S.; Seo, J.H. Production of 2,3-butanediol from xylose by engineered Saccharomyces cerevisiae. J. Biotechnol. 2014, 192, 376–382. [Google Scholar] [CrossRef]
- Guragain, Y.N.; Chitta, D.; Karanjikar, M.; Vadlani, P.V. Appropriate lignocellulosic biomass processing strategies for efficient 2,3-butanediol production from biomass-derived sugars using Bacillus licheniformis DSM 8785. Food Bioprod. Process. 2017, 104, 147–158. [Google Scholar] [CrossRef]
- Ling, H.Z.; Cheng, K.K.; Ge, J.P.; Ping, W.X. Corncob Mild Alkaline Pretreatment for High 2,3-Butanediol Production by Spent Liquor Recycle Process. Bioenergy Res. 2017, 10, 566–574. [Google Scholar] [CrossRef]
- Saratale, R.G.; Shin, H.S.; Ghodake, G.S.; Kumar, G.; Oh, M.K.; Saratale, G.D. Combined effect of inorganic salts with calcium peroxide pretreatment for kenaf core biomass and their utilization for 2,3-butanediol production. Bioresour. Technol. 2018, 258, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.W.; Jang, S.H.; Kim, Y.J.; Chang, Y.K.; Jeong, K.J. Engineering of Klebsiella oxytoca for production of 2,3-butanediol using mixed sugars derived from lignocellulosic hydrolysates. GCB Bioenergy 2020, 12, 275–286. [Google Scholar] [CrossRef] [Green Version]
- Narisetty, V.; Amraoui, Y.; Abdullah, A.; Ahmad, E.; Agrawal, D.; Parameswaran, B.; Pandey, A.; Goel, S.; Kumar, V. Bioresource Technology High yield recovery of 2,3-butanediol from fermented broth accumulated on xylose rich sugarcane bagasse hydrolysate using aqueous two-phase extraction system. Bioresour. Technol. 2021, 337, 125463. [Google Scholar] [CrossRef]
- Amraoui, Y.; Prabhu, A.A.; Narisetty, V.; Coulon, F.; Kumar Chandel, A.; Willoughby, N.; Jacob, S.; Koutinas, A.; Kumar, V. Enhanced 2,3-Butanediol production by mutant Enterobacter ludwigii using Brewers’ spent grain hydrolysate: Process optimization for a pragmatic biorefinery loom. Chem. Eng. J. 2022, 427, 130851. [Google Scholar] [CrossRef]
- van Haveren, J.; Scott, E.L.; Sanders, J. Bulk chemicals from biomass. Biofuels Bioprod. Biorefining 2007, 2, 41–57. [Google Scholar] [CrossRef]
- Nguyen, N.T.T.; Matei-Rutkovska, F.; Huchede, M.; Jaillardon, K.; Qingyi, G.; Michel, C.; Millet, J.M.M. Production of 1,3-butadiene in one step catalytic dehydration of 2,3-butanediol. Catal. Today 2019, 323, 62–68. [Google Scholar] [CrossRef]
- Sun, D.; Li, Y.; Yang, C.; Su, Y.; Yamada, Y.; Sato, S. Production of 1,3-butadiene from biomass-derived C4 alcohols. Fuel Process. Technol. 2020, 197, 106193. [Google Scholar] [CrossRef]
- Tinôco, D.; Borschiver, S.; Coutinho, P.L.; Freire, D.M.G. Technological development of the bio-based 2,3-butanediol process. Biofuels Bioprod. Biorefining 2021, 15, 357–376. [Google Scholar] [CrossRef]
- Liakou, V.; Pateraki, C.; Palaiogeorgou, A.M.; Kopsahelis, N.; Machado de Castro, A.; Guimarães Freire, D.M.; Nychas, G.J.E.; Papanikolaou, S.; Koutinas, A. Valorisation of fruit and vegetable waste from open markets for the production of 2,3-butanediol. Food Bioprod. Process. 2018, 108, 27–36. [Google Scholar] [CrossRef]
- Morschbacker, A. Bio-ethanol based ethylene. Polym. Rev. 2009, 49, 79–84. [Google Scholar] [CrossRef]
- Domínguez-Bocanegra, A.R.; Torres-Muñoz, J.A.; López, R.A. Production of Bioethanol from agro-industrial wastes. Fuel 2015, 149, 85–89. [Google Scholar] [CrossRef]
- Alvarez-Guzmán, C.L.; Balderas-Hernández, V.E.; De Leon-Rodriguez, A. Coproduction of hydrogen, ethanol and 2,3-butanediol from agro-industrial residues by the Antarctic psychrophilic GA0F bacterium. Int. J. Hydrogen Energy 2020, 45, 26179–26187. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Machado, E.M.S.; Carneiro, L.M.; Teixeira, J.A. Sugars metabolism and ethanol production by different yeast strains from coffee industry wastes hydrolysates. Appl. Energy 2012, 92, 763–768. [Google Scholar] [CrossRef] [Green Version]
- Rocha, M.V.P.; de Matos, L.J.B.L.; de Lima, L.P.; da Silva Figueiredo, P.M.; Lucena, I.L.; Fernandes, F.A.N.; Gonçalves, L.R.B. Ultrasound-assisted production of biodiesel and ethanol from spent coffee grounds. Bioresour. Technol. 2014, 167, 343–348. [Google Scholar] [CrossRef]
- Barampouti, E.M.; Grammatikos, C.; Stoumpou, V.; Malamis, D.; Mai, S. Emerging Synergies on the Co-treatment of Spent Coffee Grounds and Brewer’s Spent Grains for Ethanol Production. Waste Biomass Valoriz. 2021, 6, 1–15. [Google Scholar] [CrossRef]
- Ruta, L.L.; Farcasanu, I.C. Coffee and yeasts: From flavor to biotechnology. Fermentation 2021, 7, 9. [Google Scholar] [CrossRef]
- Bechthold, I.; Bretz, K.; Kabasci, S.; Kopitzky, R.; Springer, A. Succinic acid: A new platform chemical for biobased polymers from renewable resources. Chem. Eng. Technol. 2008, 31, 647–654. [Google Scholar] [CrossRef]
- Merli, G.; Becci, A.; Amato, A.; Beolchini, F. Acetic acid bioproduction: The technological innovation change. Sci. Total Environ. 2021, 798, 149292. [Google Scholar] [CrossRef]
- Yan, Q.; Zhao, T.; Bai, Y.; Zhang, F.; Yang, W. Precipitation polymerization in acetic acid: Study of the solvent effect on the morphology of poly(divinylbenzene). J. Phys. Chem. B 2009, 113, 3008–3014. [Google Scholar] [CrossRef]
- Abdel-Rahman, M.A.; Tashiro, Y.; Sonomoto, K. Recent advances in lactic acid production by microbial fermentation processes. Biotechnol. Adv. 2013, 31, 877–902. [Google Scholar] [CrossRef]
- Eş, I.; Mousavi Khaneghah, A.; Barba, F.J.; Saraiva, J.A.; Sant’Ana, A.S.; Hashemi, S.M.B. Recent advancements in lactic acid production-a review. Food Res. Int. 2018, 107, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Seah, R.H.; Abdul Rahaman, M.S.; Lu, Y.; Liu, S.Q. Concurrent inoculations of Oenococcus oeni and Lachancea thermotolerans: Impacts on non-volatile and volatile components of spent coffee grounds hydrolysates. LWT 2021, 148, 111795. [Google Scholar] [CrossRef]
- Lee, K.H.; Jang, Y.W.; Lee, J.; Kim, S.; Park, C.; Yoo, H.Y. Statistical optimization of alkali pretreatment to improve sugars recovery from spent coffee grounds and utilization in lactic acid fermentation. Processes 2021, 9, 494. [Google Scholar] [CrossRef]
- Kim, J.W.; Jang, J.H.; Yeo, H.J.; Seol, J.; Kim, S.R.; Jung, Y.H. Lactic Acid Production from a Whole Slurry of Acid-Pretreated Spent Coffee Grounds by Engineered Saccharomyces cerevisiae. Appl. Biochem. Biotechnol. 2019, 189, 206–216. [Google Scholar] [CrossRef]
- Adeleye, A.T.; Odoh, C.K.; Enudi, O.C.; Banjoko, O.O.; Osiboye, O.O.; Toluwalope Odediran, E.; Louis, H. Sustainable synthesis and applications of polyhydroxyalkanoates (PHAs) from biomass. Process Biochem. 2020, 96, 174–193. [Google Scholar] [CrossRef]
- Li, M.; Wilkins, M.R. Recent advances in polyhydroxyalkanoate production: Feedstocks, strains and process developments. Int. J. Biol. Macromol. 2020, 156, 691–703. [Google Scholar] [CrossRef]
- Obruca, S.; Benesova, P.; Marsalek, L.; Marova, I. Use of lignocellulosic materials for PHA production. Chem. Biochem. Eng. Q. 2015, 29, 135–144. [Google Scholar] [CrossRef]
- Saratale, R.G.; Cho, S.K.; Saratale, G.D.; Kadam, A.A.; Ghodake, G.S.; Kumar, M.; Bharagava, R.N.; Kumar, G.; Kim, D.S.; Mulla, S.I.; et al. A comprehensive overview and recent advances on polyhydroxyalkanoates (PHA) production using various organic waste streams. Bioresour. Technol. 2021, 325, 124685. [Google Scholar] [CrossRef]
- Cruz, M.V.; Paiva, A.; Lisboa, P.; Freitas, F.; Alves, V.D.; Simões, P.; Barreiros, S.; Reis, M.A.M. Production of polyhydroxyalkanoates from spent coffee grounds oil obtained by supercritical fluid extraction technology. Bioresour. Technol. 2014, 157, 360–363. [Google Scholar] [CrossRef]
- Kovalcik, A.; Kucera, D.; Matouskova, P.; Pernicova, I.; Obruca, S.; Kalina, M.; Enev, V.; Marova, I. Influence of removal of microbial inhibitors on PHA production from spent coffee grounds employing Halomonas halophila. J. Environ. Chem. Eng. 2018, 6, 3495–3501. [Google Scholar] [CrossRef]
- Obruca, S.; Benesova, P.; Petrik, S.; Oborna, J.; Prikryl, R.; Marova, I. Production of polyhydroxyalkanoates using hydrolysate of spent coffee grounds. Process Biochem. 2014, 49, 1409–1414. [Google Scholar] [CrossRef]
- Sirohi, R.; Prakash Pandey, J.; Kumar Gaur, V.; Gnansounou, E.; Sindhu, R. Critical overview of biomass feedstocks as sustainable substrates for the production of polyhydroxybutyrate (PHB). Bioresour. Technol. 2020, 311, 123536. [Google Scholar] [CrossRef]
- Tomizawa, S.; Chuah, J.A.; Matsumoto, K.; Doi, Y.; Numata, K. Understanding the limitations in the biosynthesis of polyhydroxyalkanoate (PHA) from lignin derivatives. ACS Sustain. Chem. Eng. 2014, 2, 1106–1113. [Google Scholar] [CrossRef]
- Khatami, K.; Perez-Zabaleta, M.; Owusu-Agyeman, I.; Cetecioglu, Z. Waste to bioplastics: How close are we to sustainable polyhydroxyalkanoates production? Waste Manag. 2021, 119, 374–388. [Google Scholar] [CrossRef]
- Gregory, G.L.; Lopez-Vidal, E.M.; Buchard, A. Polymers from sugars: Cyclic monomer synthesis, ring-opening polymerisation, material properties and applications. Chem. Commun. 2017, 53, 2198–2217. [Google Scholar] [CrossRef] [Green Version]
- Galbis, J.A.; García-Martín, M.D.G.; De Paz, M.V.; Galbis, E. Synthetic Polymers from Sugar-Based Monomers. Chem. Rev. 2016, 116, 1600–1636. [Google Scholar] [CrossRef]
- Zhang, Y.; Chan, J.W.; Moretti, A.; Uhrich, K.E. Designing polymers with sugar-based advantages for bioactive delivery applications. J. Control. Release 2015, 219, 355–368. [Google Scholar] [CrossRef] [Green Version]
- Zakharova, E.; De Ilarduya, A.M.; León, S.; Muñoz-Guerra, S. Sugar-based bicyclic monomers for aliphatic polyesters: A comparative appraisal of acetalized alditols and isosorbide. Des. Monomers Polym. 2017, 20, 157–166. [Google Scholar] [CrossRef]
- Gregory, G.L.; Jenisch, L.M.; Charles, B.; Kociok-Köhn, G.; Buchard, A. Polymers from sugars and CO2: Synthesis and polymerization of a d-mannose-based cyclic carbonate. Macromolecules 2016, 49, 7165–7169. [Google Scholar] [CrossRef] [Green Version]
- Lu, M.Y.; Surányi, A.; Viskolcz, B.; Fiser, B. Molecular design of sugar-based polyurethanes. Croat. Chem. Acta 2018, 91, 299–307. [Google Scholar] [CrossRef]
- Sendijarevic, I.; Pietrzyk, K.W.; Schiffman, C.M.; Sendijarevic, V.; Kiziltas, A.; Mielewski, D. Polyol from spent coffee grounds: Performance in a model pour-in-place rigid polyurethane foam system. J. Cell. Plast. 2020, 56, 630–645. [Google Scholar] [CrossRef]
- Gama, N.V.; Soares, B.; Freire, C.S.R.; Silva, R.; Neto, C.P.; Barros-Timmons, A.; Ferreira, A. Bio-based polyurethane foams toward applications beyond thermal insulation. Mater. Des. 2015, 76, 77–85. [Google Scholar] [CrossRef]
- Gama, N.; Silva, R.; Carvalho, A.P.O.; Ferreira, A.; Barros-Timmons, A. Sound absorption properties of polyurethane foams derived from crude glycerol and liquefied coffee grounds polyol. Polym. Test. 2017, 62, 13–22. [Google Scholar] [CrossRef]
- Coelho, G.O.; Batista, M.J.A.; Ávila, A.F.; Franca, A.S.; Oliveira, L.S. Development and characterization of biopolymeric films of galactomannans recovered from spent coffee grounds. J. Food Eng. 2020, 289. [Google Scholar] [CrossRef]
- Abdullah, M.; Koc, A.B. Oil removal from waste coffee grounds using two-phase solvent extraction enhanced with ultrasonication. Renew. Energy 2013, 50, 965–970. [Google Scholar] [CrossRef]
- Cubas, A.L.V.; Machado, M.M.; Bianchet, R.T.; da Costa Hermann, K.A.; Bork, J.A.; Debacher, N.A.; Lins, E.F.; Maraschin, M.; Coelho, D.S.; Moecke, E.H.S. Oil extraction from spent coffee grounds assisted by non-thermal plasma. Sep. Purif. Technol. 2020, 250, 117171. [Google Scholar] [CrossRef]
- Couto, R.M.; Fernandes, J.; da Silva, M.D.R.G.; Simões, P.C. Supercritical fluid extraction of lipids from spent coffee grounds. J. Supercrit. Fluids 2009, 51, 159–166. [Google Scholar] [CrossRef]
- Araújo, M.N.; Azevedo, A.Q.P.L.; Hamerski, F.; Voll, F.A.P.; Corazza, M.L. Enhanced extraction of spent coffee grounds oil using high-pressure CO2 plus ethanol solvents. Ind. Crops Prod. 2019, 141, 111723. [Google Scholar] [CrossRef]
- Obruca, S.; Petrik, S.; Benesova, P.; Svoboda, Z.; Eremka, L.; Marova, I. Utilization of oil extracted from spent coffee grounds for sustainable production of polyhydroxyalkanoates. Appl. Microbiol. Biotechnol. 2014, 98, 5883–5890. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Kim, J.H.; Kim, M.S.; Kim, J.; Hong, J.W.; Hong, Y.G.; Kim, H.J.; Jeon, J.M.; Kim, S.H.; Ahn, J.; et al. Production of (3-hydroxybutyrate-co-3-hydroxyhexanoate) copolymer from coffee waste oil using engineered Ralstonia eutropha. Bioprocess Biosyst. Eng. 2018, 41, 229–235. [Google Scholar] [CrossRef]
- Ingram, H.R.; Winterburn, J.B. Anabolism of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) by Cupriavidus necator DSM 545 from spent coffee grounds oil. New Biotechnol. 2021, 60, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Pascault, J.; Sautereau, H.; Verdu, J.; Williams, R.J.J. Thermosetting Polymers, 1st ed.; Marcel Dekker: New York, NY, USA, 2002; ISBN 0824706706. [Google Scholar]
- Franck, A.J. Understanding Rheology of Thermosets; TA Instruments: New Castle, DE, USA, 2004. [Google Scholar]
- Hejna, A. Potential applications of by-products from the coffee industry in polymer technology—Current state and perspectives. Waste Manag. 2021, 121, 296–330. [Google Scholar] [CrossRef] [PubMed]
- Peacock, A.J. Handbook of Polyethylene: Structures, Properties, and Applications, 1st ed.; CRC Press: Boca Raton, FL, USA, 2000; ISBN 9780429180774. [Google Scholar]
- Khanam, P.N.; AlMaadeed, M.A.A. Processing and characterization of polyethylene-based composites. Adv. Manuf. Polym. Compos. Sci. 2015, 1, 63–79. [Google Scholar] [CrossRef]
- Shih, Y.F.; Kotharangannagari, V.K.; Chen, R.M. Green composites based on high density polyethylene and recycled coffee gunny: Morphology, thermal, and mechanical properties. Mod. Phys. Lett. B 2020, 34, 1–6. [Google Scholar] [CrossRef]
- León, L.D.V.E.; Escocio, V.A.; Visconte, L.L.Y.; Junior, J.C.J.; Pacheco, E.B.A.V. Rotomolding and polyethylene composites with rotomolded lignocellulosic materials: A review. J. Reinf. Plast. Compos. 2020, 39, 459–472. [Google Scholar] [CrossRef]
- Mendes, J.F.; Martins, J.T.; Manrich, A.; Luchesi, B.R.; Dantas, A.P.S.; Vanderlei, R.M.; Claro, P.C.; de Sena Neto, A.R.; Mattoso, L.H.C.; Martins, M.A. Thermo-physical and mechanical characteristics of composites based on high-density polyethylene (HDPE) e spent coffee grounds (SCG). J. Polym. Environ. 2021, 29, 2888–2900. [Google Scholar] [CrossRef]
- Tan, M.Y.; Nicholas Kuan, H.T.; Lee, M.C. Characterization of Alkaline Treatment and Fiber Content on the Physical, Thermal, and Mechanical Properties of Ground Coffee Waste/Oxobiodegradable HDPE Biocomposites. Int. J. Polym. Sci. 2017, 2017, 6258151. [Google Scholar] [CrossRef] [Green Version]
- Cestari, S.P.; Mendes, L.C. Thermal properties and morphology of high-density polyethylene filled with coffee dregs. J. Therm. Anal. Calorim. 2013, 114, 1–4. [Google Scholar] [CrossRef]
- Cestari, S.P.; Mendes, L.C.; Altstädt, V.; Mano, E.B.; Da Silva, D.F.; Keller, J.H. Crystallization kinetics of recycled high density polyethylene and coffee dregs composites. Polym. Polym. Compos. 2014, 22, 541–549. [Google Scholar] [CrossRef]
- Cestari, S.P.; Mendes, L.C.; da Silva, D.F.; Chimanowsky, J.P., Jr.; Altstädt, V.; Demchuk, V.; Lang, A.; Leonhardt, R.G.; Keller, J.H. Properties of Recycled High Density Polyethylene and Coffee Dregs Composites. Polimeros 2013, 23, 733–737. [Google Scholar] [CrossRef] [Green Version]
- Arrigo, R.; Jagdale, P.; Bartoli, M.; Tagliaferro, A.; Malucelli, G. Structure-property relationships in polyethylene-based composites filled with biochar derived from waste coffee grounds. Polymers 2019, 11, 1336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aydemir, D.; Alsan, M.; Can, A.; Altuntas, E.; Sivrikaya, H. Accelerated weathering and decay resistance of heat-treated wood reinforced polypropylene composites. Drv. Ind. 2019, 70, 279–285. [Google Scholar] [CrossRef] [Green Version]
- Luz, S.M.; Gonçalves, A.R.; Del’Arco, A.P. Mechanical behavior and microstructural analysis of sugarcane bagasse fibers reinforced polypropylene composites. Compos. Part A Appl. Sci. Manuf. 2007, 38, 1455–1461. [Google Scholar] [CrossRef]
- Badji, C.; Beigbeder, J.; Garay, H.; Bergeret, A.; Bénézet, J.C.; Desauziers, V. Correlation between artificial and natural weathering of hemp fibers reinforced polypropylene biocomposites. Polym. Degrad. Stab. 2018, 148, 117–131. [Google Scholar] [CrossRef]
- Karina, M.; Syampurwadi, A.; Satoto, R.; Irmawati, Y.; Puspitasari, T. Physical and Mechanical Properties of Recycled Polypropylene Composites Reinforced with Rice Straw Lignin. BioResources 2017, 12, 5801–5811. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Hu, W.; Zhang, Y.; Huang, L.; Zhang, J.; Tan, S.; Cai, X.; Liao, X. Effect of oil extraction on properties of spent coffee ground–plastic composites. J. Mater. Sci. 2016, 51, 10205–10214. [Google Scholar] [CrossRef]
- Chitra, N.J.; Vasanthakumari, R.; Amanulla, S. Preliminary Studies of the Effect of Coupling Agent on the Properties of Spent Coffee Grounds Polypropylene Bio-Composites. Int. J. Eng. Res. Technol. 2014, 7, 9–16. [Google Scholar]
- Karmee, S.K. A spent coffee grounds based biorefinery for the production of biofuels, biopolymers, antioxidants and biocomposites. Waste Manag. 2018, 72, 240–254. [Google Scholar] [CrossRef]
- Zarrinbakhsh, N.; Wang, T.; Rodriguez-Uribe, A.; Misra, M.; Mohanty, A.K. Characterization of Wastes and Coproducts from Coffee Industry for Composite Material Production. BioResources 2016, 11, 7637–7653. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Bo, X.; Cong, L.; Wei, L.; McDonald, A.G. Characteristics of undeinked, alkaline deinked, and neutral deinked old newspaper fibers reinforced recycled polypropylene composites. Polym. Compos. 2018, 39, 3537–3544. [Google Scholar] [CrossRef]
- Ibrahim, I.D.; Jamiru, T.; Sadiku, R.E.; Kupolati, W.K.; Agwuncha, S.C. Dependency of the Mechanical Properties of Sisal Fiber Reinforced Recycled Polypropylene Composites on Fiber Surface Treatment, Fiber Content and Nanoclay. J. Polym. Environ. 2017, 25, 427–434. [Google Scholar] [CrossRef]
- Moreno, D.D.P.; de Camargo, R.V.; dos Santos Luiz, D.; Branco, L.T.P.; Grillo, C.C.; Saron, C. Composites of Recycled Polypropylene from Cotton Swab Waste with Pyrolyzed Rice Husk. J. Polym. Environ. 2020, 29, 350–362. [Google Scholar] [CrossRef]
- De Bomfim, A.S.C.; Voorwald, H.J.C.; de Benini, K.C.C.C.; de Oliveira, D.M.; Fernandes, M.F.; Cioffi, M.O.H. Sustainable application of recycled espresso coffee capsules: Natural composite development for a home composter product. J. Clean. Prod. 2021, 297. [Google Scholar] [CrossRef]
- Akindoyo, J.O.; Beg, M.D.H.; Ghazali, S.; Islam, M.R.; Jeyaratnam, N.; Yuvaraj, A.R. Polyurethane types, synthesis and applications-a review. RSC Adv. 2016, 6, 114453–114482. [Google Scholar] [CrossRef] [Green Version]
- Funabashi, M.; Hirose, S.; Hatakeyama, T.; Hatakeyama, H. Effect of filler shape on mechanical properties of rigid polyurethane composites containing plant particles. Macromol. Symp. 2003, 197, 231–242. [Google Scholar] [CrossRef]
- Hatakeyama, H.; Hatakeyama, T. Lignin Structure, Properties, and Applications. In Advance in Poymer Science; Springer: Berlin/Heidelberg, Germany, 2010; Volume 232, pp. 1–63. ISBN 978-3-642-13629-0. [Google Scholar]
- Baek, B.S.; Park, J.W.; Lee, B.H.; Kim, H.J. Development and Application of Green Composites: Using Coffee Ground and Bamboo Flour. J. Polym. Environ. 2013, 21, 702–709. [Google Scholar] [CrossRef]
- Suaduang, N.; Ross, S.; Ross, G.M.; Pratumshat, S.; Mahasaranon, S. Effect of spent coffee grounds filler on the physical and mechanical properties of poly(lactic acid) bio-composite films. Mater. Today Proc. 2019, 17, 2104–2110. [Google Scholar] [CrossRef]
- Cacciotti, I.; Mori, S.; Cherubini, V.; Nanni, F. Eco-sustainable systems based on poly(lactic acid), diatomite and coffee grounds extract for food packaging. Int. J. Biol. Macromol. 2018, 112, 567–575. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.C.; Chen, Y.; Ning, J.; Hao, C.; Rock, M.; Amer, M.; Feng, S.; Falahati, M.; Wang, L.J.; Chen, R.K.; et al. No Such Thing as Trash: A 3D-Printable Polymer Composite Composed of Oil-Extracted Spent Coffee Grounds and Polylactic Acid with Enhanced Impact Toughness. ACS Sustain. Chem. Eng. 2019, 7, 15304–15310. [Google Scholar] [CrossRef]
- Li, S.; Shi, C.; Sun, S.; Chan, H.; Lu, H.; Nilghaz, A.; Tian, J.; Cao, R. From brown to colored: Polylactic acid composite with micro/nano-structured white spent coffee grounds for three-dimensional printing. Int. J. Biol. Macromol. 2021, 174, 300–308. [Google Scholar] [CrossRef]
- Gama, N.; Ferreira, A.; Evtuguin, D.V. New poly(lactic acid) composites produced from coffee beverage wastes. J. Appl. Polym. Sci. 2021, 139, 51434. [Google Scholar] [CrossRef]
- Xu, H.; Xie, L.; Li, J.; Hakkarainen, M. Coffee Grounds to Multifunctional Quantum Dots: Extreme Nanoenhancers of Polymer Biocomposites. ACS Appl. Mater. Interfaces 2017, 9, 27972–27983. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Lee, H.K.; Lim, E.; Song, Y.S. Synergistic effect of lignin/polypropylene as a compatibilizer in multiphase eco-composites. Compos. Sci. Technol. 2015, 118, 193–197. [Google Scholar] [CrossRef]
- Ferreira, F.V.; Cividanes, L.S.; Gouveia, R.F.; Lona, L.M.F. An overview on properties and applications of poly(butylene adipate-co-terephthalate)–PBAT based composites. Polym. Eng. Sci. 2019, 59, E7–E15. [Google Scholar] [CrossRef] [Green Version]
- Lule, Z.C.; Kim, J. Properties of economical and eco-friendly polybutylene adipate terephthalate composites loaded with surface treated coffee husk. Compos. Part A Appl. Sci. Manuf. 2021, 140, 106154. [Google Scholar] [CrossRef]
- Lule, Z.C.; Wondu, E.; Kim, J. Highly rigid, fire-resistant, and sustainable polybutylene adipate terephthalate/polybutylene succinate composites reinforced with surface-treated coffee husks. J. Clean. Prod. 2021, 315, 128095. [Google Scholar] [CrossRef]
- Sarasini, F.; Tirillò, J.; Zuorro, A.; Maffei, G.; Lavecchia, R.; Puglia, D.; Dominici, F.; Luzi, F.; Valente, T.; Torre, L. Recycling coffee silverskin in sustainable composites based on a poly(butylene adipate-co-terephthalate)/poly(3-hydroxybutyrate-co-3-hydroxyvalerate) matrix. Ind. Crops Prod. 2018, 118, 311–320. [Google Scholar] [CrossRef]
- Sarasini, F.; Luzi, F.; Dominici, F.; Maffei, G.; Iannone, A.; Zuorro, A.; Lavecchia, R.; Torre, L.; Carbonell-Verdu, A.; Balart, R.; et al. Effect of different compatibilizers on sustainable composites based on a PHBV/PBAT matrix filled with coffee silverskin. Polymers 2018, 10, 1256. [Google Scholar] [CrossRef] [Green Version]
- Moustafa, H.; Guizani, C.; Dufresne, A. Sustainable biodegradable coffee grounds filler and its effect on the hydrophobicity, mechanical and thermal properties of biodegradable PBAT composites. J. Appl. Polym. Sci. 2017, 134, 1–11. [Google Scholar] [CrossRef]
- Moustafa, H.; Guizani, C.; Dupont, C.; Martin, V.; Jeguirim, M.; Dufresne, A. Utilization of torrefied coffee grounds as reinforcing agent to produce high-quality biodegradable PBAT composites for food packaging applications. ACS Sustain. Chem. Eng. 2017, 5, 1906–1916. [Google Scholar] [CrossRef]
- Nagarkar, R.; Patel, J. Polyvinyl Alcohol: A Comprehensive Study. Acta Sci. Pharm. Sci. 2019, 3, 34–44. [Google Scholar]
- Lessa, E.F.; Nunes, M.L.; Fajardo, A.R. Chitosan/waste coffee-grounds composite: An efficient and eco-friendly adsorbent for removal of pharmaceutical contaminants from water. Carbohydr. Polym. 2018, 189, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Le, V.T.; Pham, T.M.; Doan, V.D.; Lebedeva, O.E.; Nguyen, H.T. Removal of Pb(ii) ions from aqueous solution using a novel composite adsorbent of Fe3o4/PVA/spent coffee grounds. Sep. Sci. Technol. 2019, 54, 3070–3081. [Google Scholar] [CrossRef]
- Minh, P.T.; Thuan, L. Van Investigation of sorption mechanism of methylene blue, congo red and tannic acid from aqueous solutions onto magnetic composite sorbent obtained from alkaline pretreated spent coffee grounds. BIO Web Conf. 2021, 30, 02008. [Google Scholar] [CrossRef]
- Kanai, N.; Honda, T.; Yoshihara, N.; Oyama, T.; Naito, A.; Ueda, K.; Kawamura, I. Structural characterization of cellulose nanofibers isolated from spent coffee grounds and their composite films with poly(vinyl alcohol): A new non-wood source. Cellulose 2020, 27, 5017–5028. [Google Scholar] [CrossRef]
- Lee, H.K.; Park, Y.G.; Jeong, T.; Song, Y.S. Green nanocomposites filled with spent coffee grounds. J. Appl. Polym. Sci. 2015, 132, 2–7. [Google Scholar] [CrossRef]
- Ounkaew, A.; Kasemsiri, P.; Kamwilaisak, K.; Saengprachatanarug, K.; Mongkolthanaruk, W.; Souvanh, M.; Pongsa, U.; Chindaprasirt, P. Polyvinyl Alcohol (PVA)/Starch Bioactive Packaging Film Enriched with Antioxidants from Spent Coffee Ground and Citric Acid. J. Polym. Environ. 2018, 26, 3762–3772. [Google Scholar] [CrossRef]
- Muniappan, A.; Srinivasan, R.; Sai Sandeep, M.V.V.; Senthilkumar, N.; Senthiil, P.V. Mode-1 fracture toughness analysis of coffee bean powder reinforced polymer composite. Mater. Today Proc. 2020, 21, 537–542. [Google Scholar] [CrossRef]
- Nguyen, T.A.; Nguyen, Q.T. Hybrid Biocomposites Based on Used Coffee Grounds and Epoxy Resin: Mechanical Properties and Fire Resistance. Int. J. Chem. Eng. 2021, 2021, 1919344. [Google Scholar] [CrossRef]
- Alhelal, A.; Mohammed, Z.; Jeelani, S.; Rangari, V.K. 3D printing of spent coffee ground derived biochar reinforced epoxy composites. J. Compos. Mater. 2021, 55, 3651–3660. [Google Scholar] [CrossRef]
- Giorcelli, M.; Bartoli, M. Development of coffee biochar filler for the production of electrical conductive reinforced plastic. Polymers 2019, 11, 1916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leow, Y.; Yew, P.Y.M.; Chee, P.L.; Loh, X.J.; Kai, D. Recycling of spent coffee grounds for useful extracts and green composites. RSC Adv. 2021, 11, 2682–2692. [Google Scholar] [CrossRef]
- Tellers, J.; Willems, P.; Tjeerdsma, B.; Sbirrazzuoli, N.; Guigo, N. Spent Coffee Grounds as Property Enhancing Filler in a Wholly Bio-Based Epoxy Resin. Macromol. Mater. Eng. 2021, 306, 1–10. [Google Scholar] [CrossRef]
- Vahabi, H.; Jouyandeh, M.; Parpaite, T.; Saeb, M.R.; Ramakrishna, S. Coffee wastes as sustainable flame retardants for polymer materials. Coatings 2021, 11, 1021. [Google Scholar] [CrossRef]
- Pajtášová, M.; Ondrušová, D.; Janík, R.; Mičicová, Z.; Pecušová, B.; Labaj, I.; Kohutiar, M.; Moricová, K. Using of alternative fillers based on the waste and its effect on the rubber properties. MATEC Web Conf. 2019, 254, 04010. [Google Scholar] [CrossRef]
- Siriwong, C.; Boopasiri, S.; Jantarapibun, V.; Kongsook, B.; Pattanawanidchai, S.; Sae-Oui, P. Properties of natural rubber filled with untreated and treated spent coffee grounds. J. Appl. Polym. Sci. 2018, 135, 1–9. [Google Scholar] [CrossRef]
- Boopasiri, S.; Sae-Oui, P.; Lundee, S.; Takaewnoi, S.; Siriwong, C. Reinforcing Efficiency of Pyrolyzed Spent Coffee Ground in Styrene-Butadiene Rubber. Macromol. Res. 2021, 29, 597–604. [Google Scholar] [CrossRef]
- Stylianou, M.; Agapiou, A.; Omirou, M.; Vyrides, I.; Ioannides, I.M.; Maratheftis, G.; Fasoula, D. Converting environmental risks to benefits by using spent coffee grounds (SCG) as a valuable resource. Environ. Sci. Pollut. Res. 2018, 25, 35776–35790. [Google Scholar] [CrossRef]
- Thiagamani, S.M.K.; Nagarajan, R.; Jawaid, M.; Anumakonda, V.; Siengchin, S. Utilization of chemically treated municipal solid waste (spent coffee bean powder) as reinforcement in cellulose matrix for packaging applications. Waste Manag. 2017, 69, 445–454. [Google Scholar] [CrossRef]
- Nguyen, D.M.; Nhung, V.T.; Le Do, T.C.; Ha-Thuc, C.N.; Perre, P. Effective Synergistic Effect of Treatment and Modification on Spent Coffee Grounds for Sustainable Biobased Composites. Waste Biomass Valoriz. 2021, 13, 1339–1348. [Google Scholar] [CrossRef]




| Coproducts | Production Method | Yield 1 | References |
|---|---|---|---|
| Bioethanol | Hydrolysis of SCG fermented by S. cerevisiae | 0.26 g/g | [64] |
| Bioethanol | Hydrolysis of SCG oil extracted by ultrasound-assisted extraction fermented by S. cerevisiae | 0.5 g/g | [65] |
| Bioethanol | Hydrolysis of SCG oil and brewer’s spent grain oil, extracted by Soxhlet extraction, fermented by S. cerevisiae | 57.3% | [66] |
| Succinic acid, acetic acid, and lactic acid | Hydrolysis of SCG fermented by S. cerevisiae with yeast extract | 2.6 g/L 0.8 g/L 0.2 g/L | [12] |
| Succinic acid, acetic acid, and lactic acid | Hydrolysis of SCG fermented by O. oeni coinoculated with L. thermotolerans | 16.4 g/L 5.2 g/L 22.4 g/L | [73] |
| Lactic acid | Hydrolysis of SCG fermented by L. rhamnosus | 98% | [10] |
| Lactic acid | Hydrolysis of alkali-treated SCG fermented by L. brevis (Lb) and L. parabuchneri (Lp) | 40.1% (Lb) 55.8% (Lp) | [74] |
| Lactic acid | SCG pretreated with sulfuric acid whole slurry (s) and washed (w) and fermented by S. cerevisiae | 11.2 g/L (s) 3.4 g/L (w) | [75] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bomfim, A.S.C.d.; Oliveira, D.M.d.; Voorwald, H.J.C.; Benini, K.C.C.d.C.; Dumont, M.-J.; Rodrigue, D. Valorization of Spent Coffee Grounds as Precursors for Biopolymers and Composite Production. Polymers 2022, 14, 437. https://doi.org/10.3390/polym14030437
Bomfim ASCd, Oliveira DMd, Voorwald HJC, Benini KCCdC, Dumont M-J, Rodrigue D. Valorization of Spent Coffee Grounds as Precursors for Biopolymers and Composite Production. Polymers. 2022; 14(3):437. https://doi.org/10.3390/polym14030437
Chicago/Turabian StyleBomfim, Anne Shayene Campos de, Daniel Magalhães de Oliveira, Herman Jacobus Cornelis Voorwald, Kelly Cristina Coelho de Carvalho Benini, Marie-Josée Dumont, and Denis Rodrigue. 2022. "Valorization of Spent Coffee Grounds as Precursors for Biopolymers and Composite Production" Polymers 14, no. 3: 437. https://doi.org/10.3390/polym14030437
APA StyleBomfim, A. S. C. d., Oliveira, D. M. d., Voorwald, H. J. C., Benini, K. C. C. d. C., Dumont, M. -J., & Rodrigue, D. (2022). Valorization of Spent Coffee Grounds as Precursors for Biopolymers and Composite Production. Polymers, 14(3), 437. https://doi.org/10.3390/polym14030437