Core-Shell Magnetoactive PHB/Gelatin/Magnetite Composite Electrospun Scaffolds for Biomedical Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Characterization of Electrospun PHB/Gelatin/Magnetite Composite Scaffolds
2.2.1. Synthesis of Magnetite Particles
2.2.2. Electrospinning of Composite Fibrous Scaffolds
2.3. Characterization of the Scaffolds
2.4. Cell Cultivation and Determination of Scaffolds Cytotoxicity
3. Results
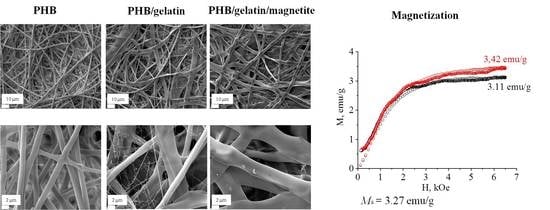
3.1. Study of the Morphology, Structure and Physico-Chemical Properties of Pure and Composite PHB Scaffolds
3.2. Determination of Cytotoxicity of the Pure and Composite Scaffolds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Manouras, T.; Vamvakaki, M. Field responsive materials: Photo-, electro-, magnetic-and ultrasound-sensitive polymers. Polym. Chem. 2017, 8, 74–96. [Google Scholar] [CrossRef]
- Adedoyin, A.A.; Ekenseair, A.K. Biomedical applications of magneto-responsive scaffolds. Nano Res. 2018, 11, 5049–5064. [Google Scholar] [CrossRef]
- Thévenot, J.; Oliveira, H.; Sandre, O.; Lecommandoux, S. Magnetic responsive polymer composite materials. Chem. Soc. Rev. 2013, 42, 7099–7116. [Google Scholar] [CrossRef] [Green Version]
- Jingcheng, L.; Reddy, V.S.; Jayathilaka, W.A.; Chinnappan, A.; Ramakrishna, S.; Ghosh, R. Intelligent Polymers, Fibers and Applications. Polymers 2021, 13, 1427. [Google Scholar] [CrossRef]
- Zhang, C.; Li, X.; Jiang, L.; Tang, D.; Xu, H.; Zhao, P.; Fu, J.; Zhou, Q.; Chen, Y. 3D Printing of Functional Magnetic Materials: From Design to Applications. Adv. Funct. Mater. 2021, 31, 2102777. [Google Scholar] [CrossRef]
- Sharifianjazi, F.; Irani, M.; Esmaeilkhanian, A.; Bazli, L.; Asl, M.S.; Jang, H.W.; Kim, S.Y.; Ramakrishna, S.; Shokouhimehr, M.; Varma, R.S. Polymer incorporated magnetic nanoparticles: Applications for magnetoresponsive targeted drug delivery. Mater. Sci. Eng. B 2021, 272, 115358. [Google Scholar] [CrossRef]
- Mmelesi, O.K.; Masunga, N.; Kuvarega, A.; Nkambule, T.T.; Mamba, B.B.; Kefeni, K.K. Cobalt ferrite nanoparticles and nanocomposites: Photocatalytic, antimicrobial activity and toxicity in water treatment. Mater. Sci. Semicond. Process. 2021, 123, 105523. [Google Scholar] [CrossRef]
- Niculescu, A.-G.; Chircov, C.; Grumezescu, A.M. Magnetite nanoparticles: Synthesis methods—A comparative review. Methods, 2021; in press. [Google Scholar] [CrossRef]
- Mai, B.T.; Fernandes, S.; Balakrishnan, P.B.; Pellegrino, T. Nanosystems based on magnetic nanoparticles and thermo-or pH-responsive polymers: An update and future perspectives. Acc. Chem. Res. 2018, 51, 999–1013. [Google Scholar] [CrossRef]
- Petcharoen, K.; Sirivat, A. Magneto-electro-responsive material based on magnetite nanoparticles/polyurethane composites. Mater. Sci. Eng. C 2016, 61, 312–323. [Google Scholar] [CrossRef]
- Liu, F.; Li, M.; Li, F.; Weng, K.; Qi, K.; Liu, C.; Ni, Q.; Tao, X.; Zhang, J.; Shao, W. Preparation and properties of PVDF/Fe3O4 nanofibers with magnetic and electret effects and their application in air filtration. Macromol. Mater. Eng. 2020, 305, 1900856. [Google Scholar] [CrossRef]
- Yao, L.; Wang, Y.; Li, Y.; Jiang, Z.; Qiu, D. Controlled preparation of Fe3O4/PLA composites and their properties. Chem. Pap. 2021, 75, 6399–6406. [Google Scholar] [CrossRef]
- Pryadko, A.; Surmeneva, M.A.; Surmenev, R.A. Review of Hybrid Materials Based on Polyhydroxyalkanoates for Tissue Engineering Applications. Polymers 2021, 13, 1738. [Google Scholar] [CrossRef] [PubMed]
- Grigore, M.E.; Grigorescu, R.M.; Iancu, L.; Ion, R.-M.; Zaharia, C.; Andrei, E.R. Methods of synthesis, properties and biomedical applications of polyhydroxyalkanoates: A review. J. Biomater. Sci. Polym. Ed. 2019, 30, 695–712. [Google Scholar] [CrossRef]
- Goonoo, N.; Bhaw-Luximon, A.; Passanha, P.; Esteves, S.R.; Jhurry, D. Third generation poly (hydroxyacid) composite scaffolds for tissue engineering. J. Biomed. Mater. Res. Part B 2017, 105, 1667–1684. [Google Scholar] [CrossRef] [PubMed]
- Degli Esposti, M.; Chiellini, F.; Bondioli, F.; Morselli, D.; Fabbri, P. Highly porous PHB-based bioactive scaffolds for bone tissue engineering by in situ synthesis of hydroxyapatite. Mater. Sci. Eng. C 2019, 100, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Babos, G.; Rydz, J.; Kawalec, M.; Klim, M.; Fodor-Kardos, A.; Trif, L.; Feczkó, T. Poly (3-hydroxybutyrate)-based nanoparticles for sorafenib and doxorubicin anticancer drug delivery. Int. J. Mol. Sci. 2020, 21, 7312. [Google Scholar] [CrossRef]
- Raza, Z.A.; Khalil, S.; Abid, S. Recent progress in development and chemical modification of poly (hydroxybutyrate)-based blends for potential medical applications. Int. J. Biol. Macromol. 2020, 160, 77–100. [Google Scholar] [CrossRef]
- Chernozem, R.; Surmeneva, M.; Shkarina, S.; Loza, K.; Epple, M.; Ulbricht, M.; Cecilia, A.; Krause, B.; Baumbach, T.; Abalymov, A. Piezoelectric 3-D fibrous poly (3-hydroxybutyrate)-based scaffolds ultrasound-mineralized with calcium carbonate for bone tissue engineering: Inorganic phase formation, osteoblast cell adhesion, and proliferation. ACS Appl. Mater. Interfaces 2019, 11, 19522–19533. [Google Scholar] [CrossRef]
- Mu, J. Three-Dimensional Hybrid Piezoelectric Polymer-Based Scaffolds for Regenerative Medicine and Biosensors. Doctoral Thesis, University of California, Riverside, CA, USA, 2020. [Google Scholar]
- Sangsanoh, P.; Supaphol, P. Poly (3-hydroxybutyrate)/magnetite composite nanofibers obtained via combined electrospinning and ammonia gas-enhancing in situ co-precipitation: Preparation and potential use in biomedical applications. Chiang Mai J. Sci. 2014, 41, 676–690. [Google Scholar]
- Ho, M.H.; Li, S.Y.; Ciou, C.Y.; Wu, T.M. The morphology and degradation behavior of electrospun poly (3-hydroxybutyrate)/Magnetite and poly (3-hydroxybutyrate-co-3-hydroxyvalerate)/Magnetite composites. J. Appl. Polym. Sci. 2014, 131, 41070. [Google Scholar] [CrossRef]
- Kokkinis, D.; Schaffner, M.; Studart, A.R. Multimaterial magnetically assisted 3D printing of composite materials. Nat. Commun. 2015, 6, 8643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sapir-Lekhovitser, Y.; Rotenberg, M.Y.; Jopp, J.; Friedman, G.; Polyak, B.; Cohen, S. Magnetically actuated tissue engineered scaffold: Insights into mechanism of physical stimulation. Nanoscale 2016, 8, 3386–3399. [Google Scholar] [CrossRef] [PubMed]
- Fuhrer, R.; Hofmann, S.; Hild, N.; Vetsch, J.R.; Herrmann, I.K.; Grass, R.N.; Stark, W.J. Pressureless mechanical induction of stem cell differentiation is dose and frequency dependent. PLoS ONE 2013, 8, e81362. [Google Scholar] [CrossRef] [PubMed]
- Shuai, C.; Cheng, Y.; Yang, W.; Feng, P.; Yang, Y.; He, C.; Qi, F.; Peng, S. Magnetically actuated bone scaffold: Microstructure, cell response and osteogenesis. Compos. Part B 2020, 192, 107986. [Google Scholar] [CrossRef]
- Lin, H.-Y.; Huang, H.-Y.; Shiue, S.-J.; Cheng, J.-K. Osteogenic effects of inductive coupling magnetism from magnetic 3D printed hydrogel scaffold. J. Magn. Magn. Mater. 2020, 504, 166680. [Google Scholar] [CrossRef]
- Fernandes, M.M.; Correia, D.M.; Ribeiro, C.; Castro, N.; Correia, V.; Lanceros-Mendez, S. Bioinspired three-dimensional magnetoactive scaffolds for bone tissue engineering. ACS Appl. Mater. Interfaces 2019, 11, 45265–45275. [Google Scholar] [CrossRef]
- Soares, P.I.; Romao, J.; Matos, R.; Silva, J.C.; Borges, J.P. Design and engineering of magneto-responsive devices for cancer theranostics: Nano to macro perspective. Prog. Mater. Sci. 2021, 116, 100742. [Google Scholar] [CrossRef]
- Adedoyin, A.A.; Ekenseair, A.K. Magneto-responsive scaffolds for tissue engineering applications. In The Road from Nanomedicine to Precision Medicine; Jenny Stanford Publishing: Singapore, 2020; pp. 559–575. [Google Scholar]
- Shrivastav, A.; Kim, H.-Y.; Kim, Y.-R. Advances in the applications of polyhydroxyalkanoate nanoparticles for novel drug delivery system. BioMed Res. Int. 2013, 2013, 581684. [Google Scholar] [CrossRef]
- Hassan, M.A.; Amara, A.A.; Abuelhamd, A.T.; Haroun, B.M. Leucocytes show improvement growth on PHA polymer surface. Pak. J. Pharm. Sci 2010, 23, 332–336. [Google Scholar]
- Naderi, P.; Zarei, M.; Karbasi, S.; Salehi, H. Evaluation of the effects of keratin on physical, mechanical and biological properties of poly (3-hydroxybutyrate) electrospun scaffold: Potential application in bone tissue engineering. Eur. Polym. J. 2020, 124, 109502. [Google Scholar] [CrossRef]
- Alipal, J.; Pu’ad, N.M.; Lee, T.; Nayan, N.; Sahari, N.; Basri, H.; Idris, M.; Abdullah, H. A review of gelatin: Properties, sources, process, applications, and commercialisation. Mater. Today Proc. 2021, 42, 240–250. [Google Scholar] [CrossRef]
- Bidone, J.; Melo, A.P.P.; Bazzo, G.C.; Carmignan, F.; Soldi, M.S.; Pires, A.T.; Lemos-Senna, E. Preparation and characterization of ibuprofen-loaded microspheres consisting of poly (3-hydroxybutyrate) and methoxy poly (ethylene glycol)-b-poly (D, L-lactide) blends or poly (3-hydroxybutyrate) and gelatin composites for controlled drug release. Mater. Sci. Eng. C 2009, 29, 588–593. [Google Scholar] [CrossRef]
- Nagiah, N.; Madhavi, L.; Anitha, R.; Srinivasan, N.T.; Sivagnanam, U.T. Electrospinning of poly (3-hydroxybutyric acid) and gelatin blended thin films: Fabrication, characterization, and application in skin regeneration. Polym. Bull. 2013, 70, 2337–2358. [Google Scholar] [CrossRef]
- Sanhueza, C.; Hermosilla, J.; Bugallo-Casal, A.; Da Silva-Candal, A.; Taboada, C.; Millan, R.; Concheiro, A.; Alvarez-Lorenzo, C.; Acevedo, F. One-step electrospun scaffold of dual-sized gelatin/poly-3-hydroxybutyrate nano/microfibers for skin regeneration in diabetic wound. Mater. Sci. Eng. C 2021, 119, 111602. [Google Scholar] [CrossRef]
- Canetti, M.; Urso, M.; Sadocco, P. Influence of the morphology and of the supermolecular structure on the enzymatic degradation of bacterial poly (3-hydroxybutyrate). Polymer 1999, 40, 2587–2594. [Google Scholar] [CrossRef]
- Chen, L.; Wang, M. Production and evaluation of biodegradable composites based on PHB–PHV copolymer. Biomaterials 2002, 23, 2631–2639. [Google Scholar] [CrossRef]
- Kreslin, V.Y.; Naiden, E. Automatic complex for a study of the characteristics of hard magnetic materials. Instrum. Exp. Tech. 2002, 45, 55–57. [Google Scholar] [CrossRef]
- Tamer, T.M.; Alsehli, M.H.; Omer, A.M.; Afifi, T.H.; Sabet, M.M.; Mohy-Eldin, M.S.; Hassan, M.A. Development of Polyvinyl Alcohol/Kaolin Sponges Stimulated by Marjoram as Hemostatic, Antibacterial, and Antioxidant Dressings for Wound Healing Promotion. Int. J. Mol. Sci. 2021, 22, 13050. [Google Scholar] [CrossRef]
- Topuz, F.; Uyar, T. Electrospinning of gelatin with tunable fiber morphology from round to flat/ribbon. Mater. Sci. Eng. C 2017, 80, 371–378. [Google Scholar] [CrossRef]
- Meng, Z.; Xu, X.; Zheng, W.; Zhou, H.; Li, L.; Zheng, Y.; Lou, X. Preparation and characterization of electrospun PLGA/gelatin nanofibers as a potential drug delivery system. Colloids Surf. B 2011, 84, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi-Mobarakeh, L.; Prabhakaran, M.P.; Morshed, M.; Nasr-Esfahani, M.-H.; Ramakrishna, S. Electrospun poly (ɛ-caprolactone)/gelatin nanofibrous scaffolds for nerve tissue engineering. Biomaterials 2008, 29, 4532–4539. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hong, R.; Li, H.; Ding, J.; Zheng, Y.; Wei, D. Simple synthesis and magnetic properties of Fe3O4/BaSO4 multi-core/shell particles. Mater. Chem. Phys. 2009, 113, 140–144. [Google Scholar] [CrossRef]
- Chowdhury, T.; D’Souza, N.; Berman, D. Electrospun Fe3O4-PVDF Nanofiber Composite Mats for Cryogenic Magnetic Sensor Applications. Textiles 2021, 1, 227–238. [Google Scholar] [CrossRef]
- Phongtamrug, S.; Tashiro, K. X-ray Crystal Structure Analysis of Poly (3-hydroxybutyrate) β-Form and the Proposition of a Mechanism of the Stress-Induced α-to-β Phase Transition. Macromolecules 2019, 52, 2995–3009. [Google Scholar] [CrossRef]
- Meade, A.D.; Lyng, F.M.; Knief, P.; Byrne, H.J. Growth substrate induced functional changes elucidated by FTIR and Raman spectroscopy in in–vitro cultured human keratinocytes. Anal. Bioanal. Chem. 2007, 387, 1717–1728. [Google Scholar] [CrossRef] [Green Version]
- Chernozem, R.V.; Romanyuk, K.N.; Grubova, I.; Chernozem, P.V.; Surmeneva, M.A.; Mukhortova, Y.R.; Wilhelm, M.; Ludwig, T.; Mathur, S.; Kholkin, A.L. Enhanced piezoresponse and surface electric potential of hybrid biodegradable polyhydroxybutyrate scaffolds functionalized with reduced graphene oxide for tissue engineering. Nano Energy 2021, 89, 106473. [Google Scholar] [CrossRef]
- Jingrun, R.; Jin, W.; Hong, S.; Nan, H. Surface modification of polyethylene terephthalate with albumin and gelatin for improvement of anticoagulation and endothelialization. Appl. Surf. Sci. 2008, 255, 263–266. [Google Scholar] [CrossRef]
- De Giglio, E.; Ditaranto, N.; Sabbatini, L. 3. Polymer surface chemistry: Characterization by XPS. In Polymer Surface Characterization; De Gruyter: Berlin, Germany, 2014; pp. 73–112. [Google Scholar]
- Nitschke, M.; Schmack, G.; Janke, A.; Simon, F.; Pleul, D.; Werner, C. Low pressure plasma treatment of poly(3-hydroxybutyrate): Toward tailored polymer surfaces for tissue engineering scaffolds. J. Biomed. Mater. Res. 2002, 59, 632–638. [Google Scholar] [CrossRef]
- Ma, W.; Yang, P.; Zhao, Y.; Huang, N. Biomimetic GelMPC Micropatterns on Titanium and Their Effects on Platelets and Endothelialization. Adv. Eng. Mater. 2018, 20, 1800624. [Google Scholar] [CrossRef]
- Yuan, S.; Xiong, G.; Roguin, A.; Teoh, S.H.; Choong, C. Amelioration of blood compatibility and endothelialization of polycaprolactone substrates by surface-initiated atom transfer radical polymerization. In Advances in Biomaterials Science and Biomedical Applications; Pignatello, R., Ed.; IntechOpen: London, UK, 2013; pp. 177–205. [Google Scholar]
- Hou, X.; Zhang, B.-L.; She, F.; Cui, Y.-L.; Shi, K.-Y.; Yao, K.-D. Surface of gelatin modified poly(L-lactic acid) film. Chin. J. Polym. Sci. 2003, 21, 277–284. [Google Scholar]
- Nagiah, N.; Madhavi, L.; Anitha, R.; Anandan, C.; Srinivasan, N.T.; Sivagnanam, U.T. Development and characterization of coaxially electrospun gelatin coated poly (3-hydroxybutyric acid) thin films as potential scaffolds for skin regeneration. Mater. Sci. Eng. C 2013, 33, 4444–4452. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, M.F.; Nuge, T.; Andriyana, A.; Ang, B.C.; Muhamad, F. Core–shell fibers: Design, roles, and controllable release strategies in tissue engineering and drug delivery. Polymers 2019, 11, 2008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, T.; Yang, H.; Yang, D.; Yu, Z.-Z. Polylactic Acid Nanofiber Scaffold Decorated with Chitosan Islandlike Topography for Bone Tissue Engineering. ACS Appl. Mater. Interfaces 2017, 9, 21094–21104. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Z.; Venugopal, J.; Huang, Z.-M.; Lim, C.T.; Ramakrishna, S. Crosslinking of the electrospun gelatin nanofibers. Polymer 2006, 47, 2911–2917. [Google Scholar] [CrossRef]
- Upadhyay, S.; Parekh, K.; Pandey, B. Influence of crystallite size on the magnetic properties of Fe3O4 nanoparticles. J. Alloys Compd. 2016, 678, 478–485. [Google Scholar] [CrossRef]
- Hao, L.; Li, L.; Wang, P.; Wang, Z.; Shi, X.; Guo, M.; Zhang, P. Synergistic osteogenesis promoted by magnetically actuated nano-mechanical stimuli. Nanoscale 2019, 11, 23423. [Google Scholar] [CrossRef]
- Cai, Q.; Shi, Y.; Shan, D.; Jia, W.; Duan, S.; Deng, X.; Yang, X. Osteogenic differentiation of MC3T3-E1 cells on poly(l-lactide)/Fe3O4 nanofibers with static magnetic field exposure. Mater. Sci. Eng. C 2015, 55, 166–173. [Google Scholar] [CrossRef]
- Yun, H.-M.; Ahn, S.-J.; Park, K.-R.; Kim, M.-J.; Kim, J.-J.; Jin, G.-Z.; Kim, H.-W.; Kim, E.-C. Magnetic nanocomposite scaffolds combined with static magnetic field in the stimulation of osteoblastic differentiation and bone formation. Biomaterials 2016, 85, 88–98. [Google Scholar] [CrossRef]
- Smit, J.; Wijn, H.P.J. Ferrites; Philips Technical Library: Eindhoven, The Netherlands, 1959. [Google Scholar]
- Rees, K.R. Cells in Culture in Toxicity Testing: A Review. J. R. Soc. Med. 1980, 73, 261–264. [Google Scholar] [CrossRef] [Green Version]
- Chernonosova, V.S.; Kvon, R.I.; Stepanova, A.O.; Larichev, Y.V.; Karpenko, A.A.; Chelobanov, B.P.; Kiseleva, E.V.; Laktionov, P.P. Human serum albumin in electrospun PCL fibers: Structure, release, and exposure on fiber surface. Polym. Adv. Technol. 2016, 28, 819–827. [Google Scholar] [CrossRef]
- Chernonosova, V.S.; Gostev, A.A.; Gao, Y.; Chesalov, Y.A.; Shutov, A.V.; Pokushalov, E.A.; Karpenko, A.A.; Laktionov, P.P. Mechanical Properties and Biological Behavior of 3D Matrices Produced by Electrospinning from Protein-Enriched Polyurethane. BioMed Res. Int. 2018, 2018, 1380606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akkas, T.; Citak, C.; Sirkecioglu, A.; Güner, F.S. Which is more effective for protein adsorption: Surface roughness, surface wettability or swelling? Case study of polyurethane films prepared from castor oil and poly(ethylene glycol). Polym. Int. 2012, 62, 1202–1209. [Google Scholar] [CrossRef]
- Vogler, E.A. Protein adsorption in three dimensions. Biomaterials 2012, 33, 1201–1237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeugolis, D.I.; Khew, S.T.; Yew, E.S.Y.; Ekaputra, A.K.; Tong, Y.W.; Yung, L.-Y.L.; Hutmacher, D.W.; Sheppar, C.; Raghunath, M. Electro-spinning of pure collagen nano-fibres—Just an expensive way to make gelatin? Biomaterials 2008, 29, 2293–2305. [Google Scholar] [CrossRef]
- Kennedy, K.M.; Bhaw-Luximon, A.; Jhurry, D. Cell-matrix mechanical interaction in electrospun polymeric scaffolds for tissue engineering: Implications for scaffold design and performance. Acta Biomater. 2017, 50, 41–55. [Google Scholar] [CrossRef]
- Chung, T.-W.; Liu, D.-Z.; Wang, S.-Y.; Wang, S.-S. Enhancement of the growth of human endothelial cells by surface roughness at nanometer scale. Biomaterials 2003, 24, 4655–4661. [Google Scholar] [CrossRef]
- Xu, C.; Yang, F.; Wang, S.; Ramakrishna, S. In vitro study of human vascular endothelial cell function on materials with various surface roughness. J. Biomed. Mater. Res. 2004, 71A, 154–161. [Google Scholar] [CrossRef]
- Di Cio, S.; Gautrot, J.E. Cell sensing of physical properties at the nanoscale: Mechanisms and control of cell adhesion and phenotype. Acta Biomater. 2016, 30, 26–48. [Google Scholar] [CrossRef]
- Lowery, J.L.; Datta, N.; Rutledge, G.C. Effect of fiber diameter, pore size and seeding method on growth of human dermal fibroblasts in electrospun poly(ɛ-caprolactone) fibrous mats. Biomaterials 2010, 31, 491–504. [Google Scholar] [CrossRef]







| Raman Shift, cm−1 | Assignments | Raman Shift, cm−1 | Assignments |
|---|---|---|---|
| 1725 | C=O stretching vibrations (crystalline phase) | 1058 | C–O stretching vibrations |
| 1460 | CH3 asymmetric bending vibrations | 953 | C–C stretching vibrations and CH3 rocking bending vibrations |
| 1443 | CH2 bending vibrations | 841 | C–COO stretching vibrations |
| 1402 | CH3 symmetric bending vibrations | 691 | C=O bending vibrations (in plane) |
| 1365 | CH bending vibrations and CH3 symmetric bending vibrations | 680 | C=O bending vibrations (out of plane) |
| 1295 | CH bending vibrations | 598 | C–CH3 and CCO bending vibrations |
| 1261 | C–O–C stretching vibrations and CH bending vibrations | 510 | C–CH3 and CCO bending vibrations |
| 1220 | COC asymmetric stretching vibrations | 367 | C–CH3 and CCO bending vibrations |
| 1101 | COC symmetric stretching vibrations | 351 | C–CH3 and CCO bending vibrations |
| Composite | Relative Atomic Concentration,% | N/C Ratio | |||
|---|---|---|---|---|---|
| C 1s | O 1s | N 1s | Others | ||
| PHB | 74 | 26 | – | – | – |
| Gelatin | 66 | 17 | 15 | >2 | 0.23 |
| PHB/gelatin | 72 | 20 | 7 | >1 | 0.10 |
| PHB/gelatin/Fe3O4 | 75 | 15 | 8 | >2 | 0.11 |
| PHB/gelatin (washed) | 74 | 24 | 2 | n/a | 0.03 |
| PHB/gelatin/Fe3O4 (washed) | 74 | 24 | 2 | n/a | 0.03 |
| Sample | Tm, °C | ΔHm, J/g | Xc,% |
|---|---|---|---|
| PHB | 177.5 | 76.3 | 52.3 |
| PHB/gelatin | 169.2 | 24.7 | 16.9 |
| PHB/gelatin/Fe3O4 | 167.9 | 13.4 | 9.2 |
| gelatin | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pryadko, A.S.; Botvin, V.V.; Mukhortova, Y.R.; Pariy, I.; Wagner, D.V.; Laktionov, P.P.; Chernonosova, V.S.; Chelobanov, B.P.; Chernozem, R.V.; Surmeneva, M.A.; et al. Core-Shell Magnetoactive PHB/Gelatin/Magnetite Composite Electrospun Scaffolds for Biomedical Applications. Polymers 2022, 14, 529. https://doi.org/10.3390/polym14030529
Pryadko AS, Botvin VV, Mukhortova YR, Pariy I, Wagner DV, Laktionov PP, Chernonosova VS, Chelobanov BP, Chernozem RV, Surmeneva MA, et al. Core-Shell Magnetoactive PHB/Gelatin/Magnetite Composite Electrospun Scaffolds for Biomedical Applications. Polymers. 2022; 14(3):529. https://doi.org/10.3390/polym14030529
Chicago/Turabian StylePryadko, Artyom S., Vladimir V. Botvin, Yulia R. Mukhortova, Igor Pariy, Dmitriy V. Wagner, Pavel P. Laktionov, Vera S. Chernonosova, Boris P. Chelobanov, Roman V. Chernozem, Maria A. Surmeneva, and et al. 2022. "Core-Shell Magnetoactive PHB/Gelatin/Magnetite Composite Electrospun Scaffolds for Biomedical Applications" Polymers 14, no. 3: 529. https://doi.org/10.3390/polym14030529
APA StylePryadko, A. S., Botvin, V. V., Mukhortova, Y. R., Pariy, I., Wagner, D. V., Laktionov, P. P., Chernonosova, V. S., Chelobanov, B. P., Chernozem, R. V., Surmeneva, M. A., Kholkin, A. L., & Surmenev, R. A. (2022). Core-Shell Magnetoactive PHB/Gelatin/Magnetite Composite Electrospun Scaffolds for Biomedical Applications. Polymers, 14(3), 529. https://doi.org/10.3390/polym14030529